
Research Article
Austin J Vet Sci & Anim Husb. 2021; 8(2): 1078.
Freezability of Goat Epididymal Sperm using Aloe vera Extract and Trehalose in Diluents
Zareie K, Farshad A*, Rostamzadeh J, Azimi G and Ariyan F
Department of Animal Science, Faculty of Agriculture, University of Kurdistan, Sanandaj, Kurdistan, Iran
*Corresponding author: Abbas Farshad, Department of Animal Science, Faculty of Agriculture, University of Kurdistan, Sanandaj, Kurdistan, Iran
Received: June 11, 2021; Accepted: July 05, 2021; Published: July 12, 2021
Abstract
Background: Cryopreservation process causes oxidative stress on sperm membranes, which in turn damages sperm organs and enzymatic activities which thereby decrease motility, functional membrane integrity and sperm fertility. Therefore, current study carried out to evaluate the effect of Aloe vera Ethanolic Extract (AEE), alone and with trehalose in diluents on cryopreserved epididymal goat sperm.
Methodology: Epididymal sperm isolated from testes with motility >70% and total morphological abnormalities <10%. The experimental treatments consist of control (no additives) and basic diluents plus 5, 10, 20 or 50 μg/ml of AEE (AEE1, AEE2, AEE3 and AEE4, respectively), tr (150 mM trehalose), tr+AEE1, tr+AEE2, tr+AEE3 and tr+AEE4.
Results: Obtained data show that the extender containing AEE3, AEE1+tr, AEE2+tr and AEE3+tr improved significantly the cryopreserved sperm. The combined treatments indicate also a decrease in MDA than control. In addition, AEE2+tr and AEE3+tr showed the lowest (P<0.05) DNA fragmentation compared to the other treatments. Extender containing AEE3+tr resulted in higher total motility and viability than the extender containing tr alone, as well as AEE1, AEE2 and AEE4 treatments.
Conclusion: The present study indicates that ethanolic extract of Aloe vera could be used for goat sperm cryopreservation. Also, it can be concluded that trehalose in combination with 20 μg/ml of Aloe vera extract can be promised cryoprotectant in goat epididymal sperm freezing.
Keywords: Antioxidant; Extender; Epididymal spermatozoa; Cryoprotectant
Background
Freezing is a method that allows long-term storage of the cell at very low temperatures [1]. Long-term preservation, establishment of genome bank, lack of need to keeping male animals, and reduction of disease transmission are the main objectives of sperm cryopreservation process [2]. Cryopreservation process includes temperature reduction, cellular dehydration, freezing and thawing. Lowering the temperature to below normal temperature will nearly stop cellular activity and reduce cellular metabolism, and after thawing, their activity will start again [3]. However, the damage to the sperm structure during the freezing-thawing process is the biggest obstacle to sperm cryopreservation leading to poor sperm quality [4]. Radical peroxyl, hydroxyl, superoxide and hydrogen peroxide are reactive oxygen samples that are highly reactive, and because of their electron deficiency, they are able to react with biological macromolecules including sugars, lipids, proteins and nucleic acids and cause sperm damage by oxidizing them [5,6]. Sperm plasma membrane contains large amounts of unsaturated fatty acids, and oxidation of unsaturated fatty acids in the sperm cell membrane results in high levels of ROS during the freezing-thawing process, which reduces membrane health and damages cellular function [7,8]. In physiological conditions, the presence of ROS is essential for normal sperm function since cell function relies on ROS [9,10]. However, during the freezing-thawing process, an imbalance between oxidizing factors and Antioxidant molecules results in oxidative stress conditions, which by increasing the production of free radicals and vitiating the antioxidant defense system, causes an increase in the rate of lipid peroxidation, DNA fragmentation, damaging apoptosis and sperm motility, and ultimately its infertility [11,12]. The aim of the new methods is to protect semen freezing, maintain sperm fertility and minimize possible damage to the sperm cell to improve its ability to fertilize oocytes [13]. There are different mechanisms to inhibit oxidative stress and reduce ROS damage during freezingthawing process, one of which is the use of antioxidants. Antioxidants are compounds that are prone to react with ROS, and by eliminating free radicals, help to maintain the homeostatic levels of radicals and prevent their harmful effects [14]. Nowadays, natural antioxidants have been studied by many researchers and plant antioxidants have been successfully used to protect sperm during cryopreservation process in domestic animals such as boar [15], sheep [16], goat [17] and bull [18]. Aloe vera (Aloe barbadensis Miller) is a plant which belongs to Liliaceae family. This plant has stiff gray-green lance-shaped leaves containing clear gel in a central mucilaginous pulp [19], and is cultivated widely in hot and dry climates in many countries [20]. Aloe vera plant, due to some properties including anti-inflammatory, antimicrobial, anticancer, wound healing, neuroprotective, anti-diabetic and antioxidant properties, is widely used as a therapeutic and medical agent [21-27]. Aloe vera is a rich source of compounds like sugars, saponins, carotenoids, flavonoids, tannins, Anthraquinone, steroids, vitamins, minerals, enzymes, polysaccharides, alkaloids, phenolic compounds, phenols and organic acids [20, 28-31]. In vivo and in vitro studies demonstrate the potential of Aloe vera as an antioxidant [32-34]. A study Hu et al. [35] shows that Aloe vera extracts had a stronger antioxidant activity than BHT and a-tocopherol. Also, Singh et al. [36] reported that Aloe vera enhances the body’s natural defense against oxidative stress by elevating the level and activity of antioxidant enzymes. Another study by Debnath et al. [37] show that Aloe vera extracts prevents lipid peroxidation, as well as DNA fragmentation by free radicals. The researchers stated that the antioxidant activity of this plant depends on its total phenolic content and that the plant can be used as a good natural antioxidant source. Rajasekaran et al. [19] stated that the consumption of Aloe vera extracts significantly increased glutathione, superoxide dismutase, catalase, glutathione peroxidase and glutathione-S-transferase in liver and kidney of STZ-induced diabetic rats. This effect indicated the antioxidant property of Aloe vera plant. Bala et al. [38] reported that the administration of Aloe vera extracts in X- ray- induced testicular damage rats, by scavenging free radicals and enhancing the antioxidant defense system prevented lipid peroxidation and apoptotic cell formation, thereby increasing testicular parameters. Also based on the study of Souza et al. [39] on using Aloe vera as a cryoprotectant in peccaries sperm extender, it was observed that sperm motility, viability and membrane integrity values were similar to egg yolk-based extender. The formation of ice crystals is one of the most important reasons for the decrease of sperm viability. Therefore, protective compounds are used in freezing extender to reduce the damage caused by ice crystals Compound sugars such as trehalose and sucrose are among these protectors [40]. Trehalose has a better protective effect on sperm during freezingthawing process compared to other sugars, and it has been used as a protector in goat [41], ram [42], buffalo [43], cattle [44], and rabbit [45] sperm cryopreservation. Trehalose is a non-penetrating disaccharide that, due to its inability to cross the plasma membrane, causes osmotic pressure, resulting in pre-freezing cell dehydration and a decrease in the formation of ice crystals within the cell. It is also of potential importance because of its influence on the stability of membrane structures during freezing [46]. To our knowledge, so far, no study has been conducted on the use of ethanolic extract of Aloe vera as an antioxidant in goat sperm freezing extender.
Therefore, the purpose of this study is to determine the antioxidant effects of ethanolic extract of Aloe vera at different levels, and to investigate the effect of a combination of ethanolic extract of Aloe vera and trehalose, to determine their possible synergistic effects through evaluation of acrosome integrity, plasma membrane functions, lipid peroxidation, DNA fragmentation and motion characteristics, after freezing-thawing process of the goat epididymal spermatozoa.
Materials and Methods
All experimental procedures used in this study have been carried out according to the international guidelines and acceptance by the Animal Care and Use Committee of the University of Kurdistan in Sanandaj, Kurdistan - Iran.
Chemicals
All chemicals used in the experiment were obtained from Sigma-Aldrich (St. Louis, MO, USA) and Merck (Darmstadt, Germany).
Preparation of aloe vera extract
The mature, healthy and fresh Aloe vera leaves were washed with fresh water and then their gel was extracted. To prepare the ethanolic extract, 100 g of the desired gel was weighed and poured into the container containing 96% ethanol. The container was placed on the shaker for 24 hours. After that, the mixture was first filtered through a clean screen cloth, and the resulting solution was then filtered through a filter paper and then concentrated by a rotary evaporator vaccum system at 40°C. The resulting substance was completely dried and turned into powder in an oven at 35°C and was finally stored at 4°C [37].
Preparation of spermatozoa
In order to do the experiment, the goat testes were obtained from a local slaughterhouse in Sanandaj and transferred to the laboratory. In order to get the spermatozoa, after several incisions in the cauda epididymis, the spermatozoa were introduced to the Tyrode lactate solution at 37°C for 15 minutes. This medium contains 100 mM NaCL, 3.1 mM KCL, 25 mM NaHCO3, 0.29 mM NaH2PO4H2O, 21.6 mM Na Lactate, 2/1 mM CaCL2 2H2O, 0.4 mM MgCL2 6H2O, 10 mM HEPES buffer, Bovine serum albumin 0.0006 g/ml, 1 mM Sodium pyruvate, 25 μg/ml Gentamycin, and Phenol red 10 mg/l [47]. Sperm suspension was centrifuged at 700 g for 10 min and then the spermatozoa were used to continue the experiment. Only sperms with total motility >70% and morphological abnormalities < 10% were chosen and used in the experiment after being pooled. The sperms were prepared with a concentration 250×106/ml. The basic extender is composed of 3.07 g Tris, 1.26 g fructose, 1.64 g citric acid in 100 ml distilled water containing 10% (v/v) egg yolk and 5% (v/v) glycerol (Maxwell and Evans 1989). The osmolality of the extenders was set on mOsm 320 and pH=7.2. The experimental treatments included basic extender with no additives (control), basic extender +150 mM of trehalose (the best trehalose concentration was obtained from previous experiments (unpublished data), basic extender +5 μg/ml concentration of Aloe vera extract, basic extender +10 μg/ml concentration of Aloe vera extract, basic extender + 20 μg/ml concentration of Aloe vera extract, basic extender +50 μg/ml concentration of Aloe vera extract, basic extender + 5 μg/ml concentration of Aloe vera extract +150 mM of tarthalose, basic extender +10 μg/ml concentration of Aloe vera extract +150 mM of tarthalose, basic extender +20 μg/ml concentration of Aloe vera extract +150 mM of trehalose, basic extender +50 μg/ ml concentration of Aloe vera extract +150 mM of trehalose. Each treatment consisted of 8 replicates. The diluted specimens were loaded into straws (0.25 mL), and after blocking the end of straws with polyvinyl chloride powder, they were put at 4°C for 3 hours. The straws were then put in nitrogen vapor at a distance of 4 cm from he liquid nitrogen level. After 15 minutes, the straws were immersed in the liquid nitrogen. The frozen straws were thawed at 37°C for 30 seconds, and were then used to evaluate the spermatozoa.
Sperm motion characteristics
Motility parameters were measured using computer-assisted sperm motility analysis system (CASA: IVOS version 12; Hamilton- Thorne Biosciences, MA, USA). Thawed semen was diluted (5 μl semen + 95 μl extender) in a Tris-based extender (without egg yolk and glycerol) and evaluated immediately after dilution. A 5-μL sample of diluted semen was put onto a pre-warmed chamber slide (Leja 4, Leja Products, Luzernestraat B.V., Holland) and sperm motility characteristics were determined with a 10× objective at 37°c. The following motility values were recorded: motility (%), progressive motility (%), VAP (average path velocity, μm/s), VSL (straight-line velocity, μm/s), VCL (curvilinear velocity, μm/s), ALH (amplitude of lateral head displacement, μm), LIN (linearity (LIN=VSL/VCL×100)), and STR (straightness (STR=VSL/VAP×100)). For each evaluation, 10 microscopic fields were analyzed to include at least 300 cells.
Sperm membrane integrity and acrosomal status
The Hypo Osmotic Swelling (HOS) test method which was described by Revell and Mrode, [48] was used to evaluate the sperm membranes integrity. This experiment was conducted by adding 10 μl of diluted sperm to 100 μl of hyposmotic solution (9g fructose +4.9g sodium citrate/1L distilled water) for 60 min at 37°C. A drop of the solution was placed on a warmed slide and was then covered with a cover slip. Two hundred spermatozoa were counted for their swelling characterized by coiled tail, and were considered as sperms with intact membranes.
Sperm acrosomal integrity was determined by the method described by Weitze [49] using formalin-citrate buffer solution (96 ml 2.9% sodium citrate, with 4 ml 37% formaldehyde). The diluted sperms were first fixed in the formalin-citrate buffer solution, and after placing a drop of this mixture on a slide, it was covered by a coverslip, and finally a total of 200 sperms were counted using a light microscope (1000×magnification) and the percentage of spermatozoa with intact acrosomes was determined.
Sperm viability, lipid peroxidation and DNA integrity
The viability of the samples was evaluated by eosin-nigrosin staining technique. Sperm suspension smears were prepared by mixing 5 μl of diluted sperms with 10 μl of eosin-nigrosin dye on a slide and spreading the dye with a second slide. After the slides were air dried, two hundred sperms were counted under a brightfield microscope (400×magnification) and the sperms that were not stained were identified as live sperms and the stained ones as dead sperms [2].
Thiobarbituric Acid (TBA) testing is routinely used to measure MDA concentrations, a tool used to estimate lipid peroxidation. For this aim, Semen samples immediately after thawing were centrifuged at 1500 g for 5 min and supernatant was separated. After mixing 1 ml of supernatants with 1 ml of EDTA (0.037 g EDTA in 10 ml distilled water), 1 ml BHT (0.2 g BHT in 10 ml ethanol) and 2 ml TCA (3 g TCA in 30 ml distilled water), we centrifuged this mixture at 1200 g for 15 minutes. This mixture was centrifuged at 1200 g for 15 min. 1 ml of supernatant of this mixture was incubated with 1 ml of TBA (0.134 g TBA in 20 ml distilled water) in a water bath at 900c for 20 min and cooled at room temperature. The absorbance was read on a spectrophotometer at 535 nm. The MDA concentrations were expressed as nmol/ml [50].
DNA fragmentation was assessed by Sperm chromatin dispersion test according to the method described by [51] with slight modification. First, 150 μl of 65% agarose was placed on a slide and covered with the coverslip. After placing the slide at 4°C for 5 min, the coverslip was removed and a mixture of 30 μl of thawed sperm sample and 70 μl of 0.7% low melting point agarose was placed on the solid agarose layer of the slide. It was then covered again by the coverslip and was air dried. The coverslip was removed and the slide was placed horizontally in acid denaturing solution (0.08 N HCl) at 37°C in darkness. After 7 minutes, the slide was placed in lysing solution (0.4 M Tris base, 0.8 M DTT, 1% SDS, 50 mM EDTA, and 2 M NaCl, pH=7.5) for 25 minutes. After that, the slide was washed with distilled water for 5 minutes and was then dehydrated in 70%, 90% and 100% ethanol respectively, each for 2 minutes, and air dried. Finally, sperm cells were stained by ethidium bromide staining solution to be assessed by fluorescence microscopy. The halo size of each cell was evaluated by the surface of the halo ÷ surface of the whole nucleoid (Figure 1).
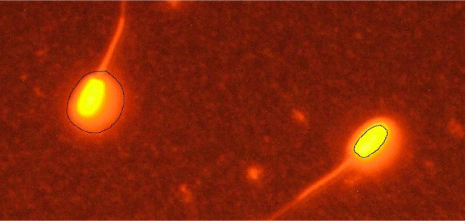
Figure 1: Demonstration of the relative halo size using digital image analyzed by fluorescence microscopy. The ethidium bromide (EtBr) stained sperm nucleoid
presents a peripheral halo of dispersed DNA loops (left) with a central core (right). The relative halo size can be calculated by the division of halo surface to whole
nucleoid surface as described by Fernandez et al. [51].
Statistical analysis
Data were analyzed using Proc GLM of SAS (version 9.1; SAS Institute, 2002, Cary, NC, USA), in a Completely Randomized Design. Orthogonal contrasts were used to compare the means and the significance level was P < 0.05. Results are shown as mean ± SE.
Results
The results in Table 1 show that the total motility in AEE3, AEE1+tr, AEE2+tr and AEE3+tr treatments significantly increased (P<0.05), compared to control treatment. AEE3+tr showed a significant increase (p<0.05) in total motility compared to AEE1, AEE2, AEE3, AEE4, tr, AEE1+tr and AEE4+tr. In addition, AEE2+tr resulted in total motility improvement compared to tr, AEE1, AEE2, AEE3, AEE4 and AEE4+tr. Progressive motility means in AEE3, AEE1+tr, AEE2+tr and AEE3+tr was significantly (p<0.05) higher than control. Also, AEE3+tr showed higher (p<0.05) percentage of Progressive motility compared to tr, AEE1, AEE2, AEE4, AEE4+tr. The results showed that the percentage of VAP were higher (p<0.05) in tr, AEE1, AEE2, AEE3, AEE1+tr, AEE2+tr and AEE3+tr compared to control. In relation to the VSL and VCL parameters, AEE3, AEE1+tr, AEE2+tr and AEE3+tr showed significant increase compared to control (P<0.05). Also, the results showed that the VCL values were higher in AEE3+tr compared to AEE4, and AEE4+tr (P<0.05). AEE3+tr resulted in an increase (P<0.05) in the ALH parameter compared to AEE4, AEE4+tr and control. Concerning the BCF parameter, AEE3, AEE1+tr, AEE2+tr and AEE3+tr treatments increased this parameter compared to control (P<0.05). The percentage of BCF also increased in AEE3+tr compared to tr, AEE1, AEE4, AEE4+tr.
Parameters
Aloe vera
Total phenolics content (mg GAE/g extract)
132.24±4.45
Total flavonoids content (mg QE/g extract)
103.81±7.69
GAE: Gallic Acid Equivalents; QE: Quercetin Equivalents.
Table 1: Analyses of the total phenolic and total flavonoid contents of Aloe vera ethanolic extract (mean ± SE; n=8).
The results of sperm viability showed that AEE3, AEE1+tr, AEE2+tr and AEE3+tr resulted in an increase (P<0.05) in sperm viability compared to control. AEE3+tr had the highest (P<0.05) viability rate than other treatment except than AEE2+tr. Also, viability in AEE1+tr and AEE2+tr treatments increased significantly (P<0.05) compared to tr, AEE1, AEE2, AEE3, AEE4 and AEE4+tr (Table 2).
Parameters
Control
AEE1
AEE2
AEE3
AEE4
tr
AEE1+tr
AEE2+tr
AEE3+tr
AEE4+tr
Total motility (%)
40.00±1.08e
41.50±1.76de
42.00±0.91cde
43.25±1.11cd
40.25±0.95de
43.00±0.82cde
44.75±1.11bc
47.00±0.82ab
48.25±0.85a
42.00±1.08cde
Progressive motility (%)
45.75±0.25d
46.75±0.63bcd
47.00±1.47bcd
48.75±0.48ab
46.00±0.41cd
47.00±0.71bcd
48.50±0.29abc
49.25±0.63ab
50.00±1.08a
46.75±1.65bcd
VAP (μm/s)
50.84±1.60c
55.76±1.62ab
55.95±1.22ab
55.99±1.56ab
51.76±0.31bc
55.75±1.25ab
57.90±1.93a
57.94±1.98a
58.48±2.99a
53.97±1.74abc
VSL (μm/s)
33.29±0.95b
36.60±0.79ab
37.01±3.12ab
37.65±0.90a
35.60±0.48ab
37.28±0.89ab
38.23±1.40a
38.63±1.95a
39.76±1.49a
36.55±0.88ab
VCL (μm/s)
121.00±3.09c
127.65±1.07abc
128.60±1.29abc
130.69±4.43ab
122.66±1.30c
128.35±1.51abc
130.49±1.90ab
133.31±5.04ab
134.38±1.80a
126.06±1.97bc
ALH (μm)
6.12±0.14b
6.37±0.24ab
6.82±0.28ab
6.97±0.05ab
6.21±0.27b
6.76±0.49ab
6.88±0.68ab
7.04±0.31ab
7.30±0.17a
6.29±0.27b
STR (%)
65.51±0.91
65.91±3.15
66.07±5.08
67.43±2.74
68.80±1.30
66.90±1.13
66.00±0.39
66.55±1.09
68.18±1.53
67.71±0.60
LIN (%)
27.51±0.16
28.69±0.82
28.81±2.50
28.93±1.32
29.04±0.65
29.05±0.55
29.32±1.20
28.94±0.41
29.65±1.33
29.04±1.08
BCF (Hz)
9.94±0.25e
10.36±0.60cde
11.04±0.75abcde
11.98±0.79abc
10.12±0.64de
10.49±0.20bcde
11.73±0.41abcd
12.21±0.67ab
12.29±0.94a
10.19±0.30de
Tr (150mM trehalose); AEE1, AEE2, AEE3 or AEE4 (5, 10, 20 or 50 μg/ml of Aloe vera ethanolic extract, respectively); average path velocity (VAP, μm/s); straight-line velocity (VSL, μm/s); curvilinear velocity (VCL, μm/s); Amplitude Of Lateral Head displacement (ALH, μm); linearity (LIN=VSL/VCL×100); straightness (STR=VSL/VAP×100); beat/cross frequency (BCF, Hz). Different superscripts (a,b,…) within the same row demonstrates significant differences (P>0.05).
Table 2: Effects of Aloe vera Ethanolic Extract (AEE) and trehalose and combinations of them on evaluated parameters in frozen-thawed goat sperm (mean ± SE; n=8).
Parameters
Control
AEE1
AEE2
AEE3
AEE4
tr
AEE1+tr
AEE2+tr
AEE3+tr
AEE4+tr
Viability (%)
44.00±1.41e
44.75±0.75de
46.75±0.48cde
47.50±1.85cd
44.50±1.76de
47.00±0.91cde
48.50±0.65bc
51.25±0.85ab
52.50±0.87a
46.50±0.87cde
Membrane integrity (%)
38.00±1.47d
40.50±0.96c
41.50±1.65c
45.25±1.89ab
38.75±1.31c
39.00±0.41c
41.25±0.85c
40.25±0.63c
47.00±1.22a
41.75±1.31bc
Acrosome statue (%)
58.50±1.19
56.50±1.55
58.25±2.84
57.75±0.25
56.75±1.65
56.50±1.32
56.50±2.53
57.75±1.60
60.50±0.65
57.75±1.31
Malondialdehyde concentration (nmol/ml)
2.74±0.22d
2.54±0.19bcd
2.47±0.14bcd
2.37±0.14abcd
2.59±0.19cd
2.38±0.10abcd
2.26±0.10abc
2.14±0.09ab
1.97±0.11a
2.52±0.14bcd
SCD stained HS/WNS (%)
1.46±0.02h
1.59±0.01fg
1.69±0.02cd
1.73±0.01bc
1.56±0.01g
1.65±0.02de
1.74±0.02b
1.79±0.02a
1.80±0.02a
1.64±0.01ef
Tr (150 mM trehalose); AEE1, AEE2, AEE3 or AEE4 (5, 10, 20 or 50 μg/ml of Aloe vera ethanolic extract, respectively). Different superscripts (a,b,…) within the same row demonstrate significant differences (P>0.05).
Table 3: Effects of Aloe vera ethanolic extract (AEE) and trehalose and combinations of them on evaluated parameters in frozen-thawed goat sperm (mean ± SE; n=8).
The HOST test results showed increase (P<0.05) in membrane integrity in all treatments compared to control. Also, results showed an increase (P<0.05) in membrane integrity in AEE3+tr compared to other treatments except than AEE3 treatment (Table 2). The results of formalin citrate test showed no significant difference (p<0.05) between treatments (Table 2).
The level of MDA, as a lipid peroxidation index, was significantly (P<0.05) lower (P<0.05) in extenders supplemented with AEE1+tr AEE2+tr, and AEE3+tr compared to the control. Moreover, the data indicated that lower (P<0.05) concentrations of MDA were detected in the extender supplemented with AEE3+tr compared to extenders supplemented with AEE1, AEE2 AEE4 and AEE4+tr.
As the results shown in Table 2 and Figure 2 Show, the Halo Surface/Whole Nucleoid Surface (HS/WNS) ratio in all treatments increased compared to the control (P<0.05). The highest (P<0.05) HS/ WNS ratio was observed in AEE2+tr and AEE3+tr treatments. This ratio was also significantly higher in AEE3 and AEE1+tr treatments compared to tr AEE1, and AEE4+tr treatments (P<0.05).
Figure 2: Effects of Aloe vera Ethanolic Extract (AEE) and trehalose and combinations of them on DNA fragmentation in frozen-thawed goat sperm. Tr (150mM
trehalose); AEE1, AEE2, AEE3 or AEE4 (5, 10, 20 or 50 μg/ml of Aloe vera ethanolic extract, respectively).
Discussion
During the semen cryopreservation process, lowering the temperature causes oxidative stress on the sperm membranes, which in turn damages sperm organs and enzymatic activities, and decreases motility, functional membrane integrity, and sperm fertility [52,53]. Some plants have effective antioxidant properties due to their bioactive compounds, including phenols and flavonoids, which can reduce the damage caused by free radicals [54]. To our knowledge, so far, no study has been conducted on the use of ethanolic extract of Aloe vera as an antioxidant in goat sperm freezing extender. Therefore, the aim of this study is to determine the antioxidant effects of different levels of ethanolic extract of Aloe vera, and to investigate the effect of a combination of ethanolic extract of Aloe vera and trehalose after freezing-thawing process of the goat epididymal spermatozoa. Our findings showed that Aloe vera extract alone or in combination with trehalose improved sperm quality. Based on our results, the use of 20 μg/ml of Aloe vera extract in the extender resulted in significant improvement in total motility, progressive motility, VSL, VCL, BCf, viability and membrane integrity compared to control treatment. The results showed that adding a 20 μg/ml of ethanolic extracts of Aloe vera to extender decreased MDA compared to control treatment. These are in agreement with the report of Najafi et al. [55] who mentioned that the addition of fennel extracts to the ram sperm freezing extender significantly increased total motility, progressive motility, membrane integrity and sperm viability parameters. Also, Azimi et al. [56] reported that the use of purslane extract in extender improved total motility, progressive motility, viability, membrane integrity and reduced lipid peroxidation of sperm after thawing. Merati and Farshad [57] indicated that the addition of ginger and echinacea extract to epididymal ram sperm freezing extender resulted in increased total motility, progressive motility, viability, membrane integrity and decreased MDA of sperm after freezing-thawing process. In another report by Zanganeh et al. [17], using rosemary extracts in goat semen extender resulted in increased total motility, progressive motility, and membrane integrity and decreased MDA of human sperm. Yong et al. [58] stated that the use of Aloe vera as a cryoprotectant for fish semen cooling increased sperm viability and motility. Also, Jasem and Nasim [59] reported that Aloe vera can increase male fertility by enhancing sperm quality. These results indicate that natural antioxidants play an important role in preventing sperm oxidation and can maintain membrane motility and integrity during cryopreservation. The precise mechanism by which Aloe vera extract enhances sperm quality is not known yet. According to the reports, this plant contains folic acid and zinc which act as antioxidants and can enhance sperm quality by reducing semen apoptosis [60,61]. Research has also shown that Aloe vera extract contains phenols [30]. Phenolic compounds are widely distributed in plants and can directly excavate free radicals. They have greater antioxidant power than synthetic antioxidants and vitamins C and E [62,63] reported that the antioxidant activity of ethanolic extract of Aloe vera well-correlated to total phenolic content. Therefore, sperm quality improvement may be due to the antioxidant role of phenolic compounds of Aloe vera. Phenolic antioxidants act as free radical terminators or metal chelators [64]. The antioxidant properties of phenolic compounds can be attributed to their ability to donate hydrogen ions [65]. The results of this experiment showed that the extender containing Aloe vera extract resulted in reduced DNA fragmentation after thawing. Similar to our results, Azimi et al. [56] reported that the addition of purslane extract to goat sperm freezing extender reduced DNA fragmentation after thawing. In addition, Ariyan et al. [66] showed that the use of Tribulus terrestris in goat extender resulted in decrease of sperm DNA fragmentation. Also, Luno et al. [67] showed that the addition of mate tea extract to pig sperm freezing extender reduced DNA fragmentation. In another study Shah et al. [18] observed that the addition of curcumin to the buffalo freezing extender increased DNA integrity after cryopreservation. But Asadmobini et al. [15] did not observe a significant effect on the integrity of postthaw sperm DNA after adding different levels of Tribulus terrestris extract to human semen freezing diluent. The exact mechanism by which Aloe vera extract reduces sperm DNA fragmentation is not known. However, research indicates that flavonoids can prevent DNA breakage and damage by electron transfer [68]. The results show that the extender containing combined treatment of AEE1+tr, AEE2+tr and AEE3+tr resulted in an improvement in total motility, progressive motility, VSL, VCL, BCF, viability, membrane and DNA integrity compared to control. Also, all combined treatments, resulted in a decrease (P<0.05) in MDA than control. In addition, our results showed that the extender containing the combined treatment of AEE3+tr showed significantly higher motility and viability than the extender containing the tr treatment alone as well as the AEE1, AEE2 and AEE4 treatments. Also, the combined treatments of AEE1+tr, AEE2+tr and AEE3+tr, compared to trehalose or the extracts alone, resulted in reduced DNA fragmentation. Wang and Dong [46] observed that the addition of trehalose in combination with glutathione to the freezing extender, compared to the addition of glutathione and trehalose alone to the extender, significantly increased the progressive motility and membrane integrity percentages. They mentioned that trehalose in combination with glutathione could better protect deer sperm during the freezing-thawing process. The experiment conducted by Daghigh-Kia et al. [69] showed that the use of rosemary in combination with glutathione led to improved postthaw quality of bull semen. These results are in agreement with our results which show that trehalose in combination with the extracts resulted in improved sperm quality compared to the addition of trehalose or the extracts alone to the extender. The mechanisms underlying the protective effect of trehalose in combination with Aloe vera extract on sperm cryopreservation have not been reported so far. However, it can be concluded from the results of this experiment that the existing compounds in the Aloe vera extracts might have worked with trehalose in a synergy.
Conclusions
Based on the results of this experiment, it can be stated that the use of ethanolic extract of Aloe vera as an antioxidant in the freezing extender of goat epididymal spermatozoa has beneficial effects on cell protection during freezing-thawing process. According to the present results, the increase in post-thaw sperm quality due to the addition of a combination of trehalose and the ethanolic extract of Aloe vera to the extender may indicate the synergistic effects of trehalose and the existing compounds in the ethanolic extract of this plant. Therefore, it can be concluded that trehalose in combination with Aloe vera ethanolic extract can be a useful cryoprotectant in goat sperm freezing. However, further research is needed to determine the precise effect of biomolecules present in Aloe vera extract as well as their antioxidant protective mechanisms in combination with trehalose during cryopreservation. However, further researches directed at the discovery of a better effectivity of cryopreservation techniques using this antioxidant such artificial insemination.
Limitations of the Study
Due to financial constraints, the availability of livestock and the lake of laboratory facilities, we were not able to use the frozen-thawed sperm in artificial insemination program. Given the existence of this condition, we could have a better analysis of the obtained results. Moreover, it is important to note that we do not know how the used antioxidants would affect the sperm from other species.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- Farshad A, Khalili B, Jafaroghli M. Effects of butylated hydroxytoluene on freezability of ram spermatozoa. Asian-Australasian J Anim Sci. 2010; 23: 1276-1281.
- Evans G, Maxwell WC. Salamons’ artificial insemination of sheep and goats. 1987.
- Barbas JP, Mascarenhas RD. Cryopreservation of domestic animal sperm cells. Cell Tiss Bank 2009; 10: 49-62.
- Watson PF. The causes of reduced fertility with cryopreserved semen. Anim Reprod Sci. 2000; 60: 481-492.
- Sikka SC. Relative impact of oxidative stress on male reproductive function. Curre Medic Chem. 2001; 8: 851-862.
- Agarwal A, Prabakaran SA, Said TM. Prevention of oxidative stress injury to sperm. J Androl. 2005; 26: 654-660.
- Kheradmand A, Babaei H, Abshenas J. Comparative evaluation of the effect of antioxidants on the chilled-stored ram semen. Iran J Vet Res. 2006; 7: 40-45.
- Uysal O, Bucak MN. Effects of oxidized glutathione, bovine serum albumin, cysteine and lycopene on the quality of frozen-thawed ram semen. Acta Vet Brno. 2007; 76: 383-390.
- Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology 1996; 48: 835-850.
- Kim S, Agca C, Agca Y. Changes in rat spermatozoa function after cooling, cryopreservation and centrifugation processes. Cryobiology 2012; 65: 215- 223.
- Armstrong JS, Rajasekaran M, Chamulitrat W, Gatti P, Hellstrom WJ, Sikka SC. Characterization of reactive oxygen species induced effects on human spermatozoa movement and energy metabolism. Free Rad Biol Med. 1999; 26: 869-880.
- Bucak MN, Sariozkan S, Tuncer PB, Sakin F, Atessahin A, Kulaksiz R, et al. The effect of antioxidants on post-thawed Angora goat (Capra hircus ancryrensis) sperm parameters, lipid peroxidation and antioxidant activities. Small Rum Res. 2010; 89: 24-30.
- Baker SS, Thomas M, Thaler CD. Sperm membrane dynamics assessed by changes in lectin fluorescence before and after capacitation. J Androl. 2004; 25: 744-751.
- Ogbuewu IP, Aladi NO, Etuk IF, Opara MN, Uchegbu MC, Okoli IC, et al. Relevance of oxygen free radicals and antioxidants in sperm production and function. J Vet Sci. 2010; 3: 138-164.
- Asadmobini A, Bakhtiari M, Khaleghi S, Esmaeili F, Mostafaei A. The effect of Tribulus terrestris extract on motility and viability of human sperms after cryopreservation. Cryobiology 2017; 75: 154-159.
- Khodaei Motlagh M, Sharafi M, Zhandi M, Mohammadi-Sangcheshmeh A, Shakeri M, Soleimani M, et al. Antioxidant effect of rosemary (Rosmarinus officinalis L.) extract in soybean lecithin-based semen extender following freeze–thawing process of ram sperm. Cryobiology 2014; 69: 217-222.
- Zanganeh Z, Zhandi M, Zare-Shahneh A, Najafi A, Nabi MM, Mohammadi- Sangcheshmeh A. Does rosemary aqueous extract improve buck semen cryopreservation. Small Rum Res. 2013; 114: 120-125.
- Shah SAH, Andrabi SMH, Qureshi IZ. Freezability of water buffalo bull (Bubalus bubalis) spermatozoa is improved with the addition of curcumin (diferuoyl methane) in semen extender. Andrology 2016; 49: 1-10.
- Rajasekaran S, Sivagnanam K, Subramanian S. Antioxidant effect of Aloe Vera gel extract in streptozotocin-induced diabetes in rat. Pharm Rep. 2005; 57: 90-96.
- Choi S, Chung MH. A review on the relationship between Aloe Vera components and their biologic effects. In Semin integ Med. 2003; 1: 53-62.
- Kim K, Kim H, Kwon J, Lee S, Kong H, Im SA, et al. Hypoglycemic and hypolipidemic effects of processed Aloe vera gel in a mouse model of noninsulin- dependent diabetes mellitus. Phytomedicine 2009; 16: 856-863.
- El-Shemy HA, Aboul-Soud MAM, Nassr-Allah AA, Aboul-Enein KM, Kabash A, Yagi A. Antitumor Properties and Modulation of Antioxidant EnzymesActivityby Aloe vera Leaf Active Principles Isolated via Supercritical Carbon Dioxide Extraction. Curr Med Chem. 2010; 17: 129-138.
- Kedarnath Kamble Kaveri M, Chimkod Vishwanath B, Patil CS. Antimicrobial Activity of Aloe Vera Leaf Extract. Int J Appl Bio Pharma Tech. 2013; 4: 286- 290.
- Hashemi SA, Madani SA, Abediankenari S. The review on properties of Aloe vera in healing of cutaneous wounds. BioMed Res Int. 2015; 2015: 714216.
- Gabriel NN, Qiang J, Ma XY, HeJ, Xu P, Liu K. Dietary Aloe vera improves plasma lipid profile, antioxidant, and hepatoprotective enzyme activities in GIFT-tilapia (Oreochromis niloticus) after Streptococcus iniae challenge. Fish Physio Bio. 2015; 41: 1321-1332.
- Yuksel Y, Guven M, Kaymaz B, Sehitoglu MH, Aras AB, Akman T, Tosu, M, Cosar M. Effects of aloe vera on spinal cord Ischemia–Reperfusion injury of rats. J Invest Surg. 2016; 29: 389-398.
- Guven M, Golge UH, Aslan E, Sehitoglu MH, Aras AB, Akman T, Cosar M. The effect of aloe vera on ischemia–Reperfusion injury of sciatic nerve in rats. Biomed Pharma. 2016; 79: 201-207.
- Hamman JH. Composition and applications of Aloe vera leaf gel. Molecules. 2008; 13: 1599-1616.
- Akaberi M, Sobhani Z, Javadi B, Sahebkar A, Emami SA. Therapeutic effects of Aloe spp. in traditional and modern medicine: a review. Biomed Pharma. 2016; 31: 759-772.
- Quispe C, Villalobos M, Borquez J, Simirgiotis M. Chemical Composition and Antioxidant Activity of Aloe vera from the Pica Oasis (Tarapac´a, Chile) by UHPLC-Q/Orbitrap/MS/MS. J Chem. 2018; 1-12.
- Lopez-Cervantes J, Sánchez-Machado DI, Cruz-Flores P, Mariscal- Domínguez MF, de la Mora-López GS, Campas-Baypoli ON. Antioxidant capacity, proximate composition, and lipid constituents of Aloe vera flowers. J Appl Res Med Arom Plan. 2018; 10: 93-98.
- Taukoorah U, Fawzi Mahomoodally M. Crude Aloe vera Gel Shows Antioxidant Propensities and Inhibits Pancreatic Lipase and Glucose Movement in vitro. Advan Pharma Sci. 2016; 2016: 3720850.
- Behmanesh MA, Najafzadehvarzi H, Poormoosavi SM. Protective Effect of Aloe vera Extract against Bisphenol A Induced Testicular Toxicity in Wistar Rats. Cell J. (Yakh). 2018; 2: 278-283.
- Sacan O, Akev N, Yanardag R. In vitro inhibitory effect of Aloe vera (L.) Burm. f. leaf extracts on the activity of some enzymes and antioxidant activity. Ind J Bio Bio. 2017; 54: 82-89.
- Hu Q, Hu Y, Xu J. Free radical-scavenging activity of Aloe vera (Aloe barbadensis Miller) extracts by supercritical carbon dioxide extraction. Food Chem .2005; 91: 85-90.
- Singh J, Koley KM, Chandrakar K, Pagrut NS. Effects of Aloe vera on dressing percentage and haemato-biochemidal parameters of broiler chickens. Vet Worl. 2013; 6: 803-814.
- Debnath T, Ghosh M, Lee YM, Nath NCD, Lee KG, Lim BO. Identification of phenolic constituents and antioxidant activity of Aloe barbadensis flower extracts. Food Agri Immun. 2018; 29: 27-38.
- Bala S, Chugh NA, Bansal SC, Garg ML, Koul A. Protective role of Aloe vera against X- ray induced testicular dysfunction. Andrologia. 2016; 49: 1-12.
- Souza ALP, Lima GL, Peixoto GCX, Silva AM, Oliveira MF, Silva AR. Use of Aloe vera–based extender for chilling and freezing collared peccary (Pecari tajacu) semen. Theriogenology. 2016; 85: 1432-1438.
- De Leeuw F, De Leeuw A, Den Daas J, Colenbrander B, Verkleij A. Effects of various cryoprotective agents and membrane-stabilizing compounds on bull sperm membrane integrity after cooling and freezing. Cryobiology. 1993; 30: 32-44.
- Khalili B, Farshad A, Zamiri MJ, Rashidi A, Fazeli P. Effects of sucrose and trehalose on the freezability of Markhoz goat spermatozoa. Asian- Australasian J Anim Sci. 2009; 22: 1614-1619.
- Inanc ME, Gungor S, Ozturk C, Korkmaz F, Bastan I, Cil B. Cholesterolloaded cyclodextrin plus trehalose improves quality of frozen-thawed ram sperm. Vet Med. 2019; 64: 118-124.
- Iqbal S, Andrabi SMH, Riaz A, Durrani AZ, Ahmad N. Trehalose improves semen antioxidant enzymes activity, post-thaw quality, and fertility in Nili Ravi buffaloes (Bubalus bubalis). Theriogenology 2016; 85: 954-959.
- El-Sheshtawy RI, Sisy GA, El-Nattat WS. Effects of different concentrations of sucrose or trehalose on the post-thawing quality of cattle bull semen. Asi Pac J Reprod. 2015; 4: 26-31.
- Zhu Z, Fan X, Pan Y, Lu Y, Zeng W. Trehalose improves rabbit sperm quality during cryopreservation. Cryobiology 2017; 75: 45-51.
- Wang Y, Dong S. Glutathione in combination with trehalose has supplementary beneficial effects on cryopreserved red deer (cervus elaphus) sperm. Am J Reprod Immunol. 2017; 77: 1-4.
- Anand M, Yadav S, Vaswani SH, Shukla P. Kinematic response of buck sperm to low-density lipoproteins in fresh diluted, short term and long-term stored semen. J Anim Res. 2017; 7: 253-257.
- Revell SG and Mrode RA. An osmotic resistance test for bovine semen. Anim Reprod Sci. 1994;36:77-86.
- Weitz KF. Unter suchungen zur Tiefgefriekonservierung von Kaninchensperma. Habil-Schr., Tieraerzh. Hochs. Honnover, Germany. 1977.
- Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. In Met Enzym. 1990; 186: 407-421.
- Fernandez JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003; 24: 59-66.
- Bucak MN, Tuncer PB, Sariozkan S, Ulutas PA. Comparison of the effects of glutamine and an amino acid solution on post-thawed ram sperm parameters, lipid peroxidation and anti-oxidant activities. Small Rum Res. 2009; 81: 13-17.
- Bucak MN, Sariozkan S, Tuncer PB, Ulutas PA, Akcadag HI. Effect of antioxidants on microscopic semen parameters, lipid peroxidation and antioxidant activities in Angora goat semen following cryopreservation. Small Rum Res. 2009; 81: 90-95.
- Ghotbabadi FS, Alizadeh A, Zadehbagheri M, Kamelmanesh MM, Shaabani M. Phytochemical composition of the essential oil, total phenolic content, antioxidant and antimicrobial activity in Iranian Satureja sahendica Bornm. at different ontogenesis conditions. J Med Plan Res. 2012; 6: 3525-3534.
- Najafi A, Daghigh Kia H, Mehdipour M, Shamsollahi M, Miller DJ. Does fennel extract ameliorate oxidative stress frozen-thawed ram sperm?. Cryobiology. 2019; 87: 47-51.
- Azimi G, Farshad A, Farzinpour A, Rostamzadeh J, Sharafi M. Evaluation of used Purslane extracts in Tris extenders on cryopreserved goat sperm. Cryobiology. 2020; 94: 40-48.
- Merati Z. Farshad A. Ginger and echinacea extracts improve the quality and fertility potential of frozen-thawed ram epididymal spermatozoa. Cryobiology 2020; 92: 138-145.
- Yong SY, Nguang SI, Kari A. Comparision between the effect of egg yolkbased extender and Aloe vera (Aloe Barbadensis)-based extender on red tilapia (Oreochromis Niloticus) sperm quality. J Fund Appl Sci. 2017; 9: 813-820.
- Jasem E, Nasim J. Spermatogenic activity Aloe Vera in adult male rat. Pharmacology online 2011; 2: 886-889.
- Pfeifer H, Conrad M, Roethlein D, Kyriakopoulos A, Brielmeier M, Bornkamm GW, Behne D. Identification of a specific sperm nuclei selenoenzyme necessary for protamine thiol cross-linking during sperm maturation. FASEB J. 2001; 15: 1236-1238.
- Shahraki A, Mojahed AS, Afshar-Goli JThe effects of hydroalcoholic extract of Aloe vera gel on spermatogenesis of adult male rats. Int J Bio. 2014; 5: 158-165.
- Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plan Sci. 1997; 2: 152-159.
- Prior RL, Cao G. Antioxidant phytochemicals in fruits and vegetables: diet and health implications. Hort Sci. 2000; 35: 588-592.
- Shahidi F, Wanasundara PK, Janitha PD. Phenolic antioxidants. Critic Rev Food Sci Nut. 1992; 32: 67-103.
- Wojdylo A, Oszmianski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007; 105: 940-949.
- Ariyan F, Farshad A, Rostamzadeh J. Protective effects of Tribulus terrestris and Cinnamomum zeylanicum extracts and trehalose added to diluents on goat epididymal sperm freezability. Cryobiology. 2021; 98: 172-180.
- Luno V, Gil L, Olaciregui M, Jerez RA, de Blas I, Hozbor F. Antioxidant effect of lemon balm (Melissa officinalis) and mate tea (Ilex paraguensys) on quality, lipid peroxidation and DNA oxidation of cryopreserved boar epididymal spermatozoa. Andrologia. 2015; 47: 1004-1011.
- Zribi N, Chakroun NF, Abdallah FB, Elleuch H, Sellami A, Gargouri J, et al. Effect of freezing–thawing process and quercetin on human sperm survival and DNA integrity. Cryobiology. 2012; 65: 326-331.
- Daghigh-Kia H, Olfati-Karaji R, Hoseinkhani A, Ashrafi I. Effect of rosemary (Rosmarinus officinalis) extracts and glutathione antioxidants on bull semen quality after cryopreservation. Span J Agri Res. 2014; 12: 98-105.