
Research Article
Austin J Vet Sci & Anim Husb. 2022; 9(4): 1101.
A Study on Trypanocidal Drug Resistance: A Risk on Animal Health and Production in Jawi District of North West Ethiopia
Ashagrie T1*, Zewde D1, Rufael T1, Mekonnen G1, Kefyalew H2, Selamu A3, Yenew A4 and Kassahun A4
1Animal Health Institute, Ethiopia
2Bahirdar Regional Veterinary Laboratory, Bahirdar, Ethiopia
3Finote selam Tsetse and Trypanosomiasis Investigation and Control Center, Finote selam, Ethiopia
4Jawi District Animal Health and Development Office, Jawi, Ethiopia
*Corresponding author: Tigist Ashagrie, Animal Health Institute, Ethiopia
Received: August 05, 2022; Accepted: September 02, Published: September 09, 2022
Abstract
The study was conducted from February to June, 2021, with the objective to assess the occurrence of trypanocidal drug resistance in naturally infected bos indicus breed of cattle in hot spot villages of Jawi district, North Western Ethiopia. An initial cross-sectional prevalence study was conducted in four villages with the aim of finding at least 10% trypanosome parasite prevalence for further longitudinal drug susceptibility trial. Accordingly, out of 464 cattle examined from all villages, 53 (11.4%) animals were trypanosome-positive using a Buffy coat test method. For the appropriateness of monitoring and follow up of animals subjected to drug susceptibility trial and on the basis of village’s adjacency, the areas were merged in two sites (i.e. Achari and Kurey peasant associations). A 28-day field protocol study was used to estimate proportion of resistance to a recommended dose of 0.5 mg/kg Body Weight (bw) for Isometamidium Chloride (ISM) and 3.5mg/kg bw for Diminazene Aceturate (DIM). In this study, 52 trypanosome positive cattle were ear-tagged and allocated into two treatment groups. Group I, which consists 27 animals was treated with 7% solution of 3.5mg/kg bw DIM and the second group having 25 animals were subjected to 1% solution of 0.5 mg/kg bw ISM with 25 trypanosome free animals as a control. Before the actual treatment begin all animals grouped under treatment and control groups were subjected to deltamethrin 1% pour-on at day 0 on the animals back midline. Animals under trial were monitored for the status of trypanosomes and Packed Cell Volume (PCV) levels on days 14 and 28 post treatment. A treatment failure rate of 40% (10/25) forcattle’s treated with ISM was observed on day 28; whereas, 48.1% (13/27) DA treated animals on day 14 post-treatments were failed to clear the parasites. The results of the study confirmed the presence of drug resistance to the maximum recommended doses of ISM and DIM in the study district. Of all ISM and DIM treatment failures T. congolense accounted for 77.8% (7/9), 53.8% (7/13)); T. vivax (0%, 20%) and T. teleri (100%, 100%) respectively. Drug resistance has indeed been a considerable threat in each village of the study sites. DIM treated trypanosomepositive cattle showed a significantly increased (p < 0.001) PCV% from 19.9 % at day 0 to 21.2 % at day 14 and eventually to 23.8% at day 28. Similarly, ISM treated animals considerably increased (p<0.001) PCV% from 21.5% at day 0 to 23.9% at day 14 and then to 25.0% at day 28. However, the PCV% value for the negative control has no statistical variation (p=0.662) among the various days under investigation. Rational use of trypanocidal drugs and control of co-infections to exploit self-cure against resistant trypanosome populations are recommended. Furthermore, further study using advanced molecular techniques to explore possible pathways on development of drug resistance could be necessary.
Keywords: Bovine; Buffy coat; Drug resistance; Ethiopia; Trypanosomosis
Introduction
Form the prevalent diseases of animal parasitism trypanosomiasis has a major complication to the development of animal health sector. It can affect health, reduce growth rate and birth weight, cause reproductive problems and reduce the usefulness of carcasses. Parasitic diseases are well known as main causes of economic losses in livestock worldwide [1,2]. Economic loss from parasitic disease is due to mortality, morbidity, reduced milk production and indirect impact on crop production through the availability and cost of animals that provide traction power. Among the existing parasitic diseases in Africa, Trypanosomosis (AAT) or Nagana is a daunting major problem in cattle and other ruminants [3].
Different trypanocidal drugs are used to prevent and treat trypanosomosis. In Africa about 35 million doses of drugs are used in each year, about 50-70 million animals at risk from trypanosomosis [4]. Since most of trypanocidal drugs have been in use for more than half a century, they can cause the appearance of the drug resistant strain of trypanosomes [5]. Trypanocidal drug resistance has been reported from 21 African countries. In ten of the 21 African countries, the occurrence of drug resistance to Diminazine Aceturate (DA), Isometamidium Chloride (ISM) and Homodium Bromide (HOM) has been reported [6]. Various researchers across East Africa reported resistance to currently available trypanocidal drugs and the approaches to the mechanisms of drug resistance in trypanosomes have been extensively reviewed [7]. In Ethiopia, ISM and DA have been used for several years. Trypanocidal resistance particularly against T. congolense infection was reported from Ghibe valley as early as in 1989 [8,9]. It was found that 11 of the 12 isolates tested were resistant in cattle to recommended doses of isometamidium and homidium. Since then, several other reports have emerged substantiating the widespread occurrence of trypanocidal drug resistance in many parts of the country [10-14].
A longitudinal study was followed for a 28 day field protocol which was a ‘Block treatment’ approach [5], that have been proposed to enable identification of probable resistance in the field, where by cattle in a particular location are split into control and treatment groups and followed for the detection of parasitemia. The two groups, naturally infected by trypanosome, were tested for trypanosomes positivity using Buffy coat technique [15]. Based on the protocol by [16], trypanocidal drug resistance study map ping have been documented by several epidemiological studies. [17] From Kénédougou Province of Burkina Faso, [18] from Eastern Zambia, [11] from Ethiopia, [19] from Guinea and south-eastern Mali and [20] from Ghana are some examples. In this regard, use of trypanocidal drugs must be carefully monitored and trypanosome populations need to be screened regularly for the appearance of drug resistant parasites. Based on the above concepts, we used to conduct in vivo resistivity test based on [21] in a 4 - week longitudinal survey. This was effective in areas where trypanosomosis risk was high (prevalence is >10%) [22]. for that reason, trypanosome drug resistance study was conducted in Jawi district where tsetse transmitted trypanosomosis become a serious threat for livestock production and agricultural activity [23,24]. The objective of the study in this district was todetermine the prevalence and assess magnitude of trypanocidal susceptibility in naturally infected cattle of the district.
Materials and Method
Study Area
The current study was conducted on purposively selected four village of Jawi district in Awi zone. The selection criteria was based on the extent of the existing problems, the complaints of farmers and the level of medium to high tsetse challenge in the area from the report of the field veterinarian as well as based on our recent parasitological findings in the area. Jawilies within the geographical location of 360 - 370E and 100380 to 110 30`N. The district has an altitude range from 648 to 1200 m.a.s.l. It has a climate which can be described as tropical with winter dry season. The agro-ecological area of the district has a warm and humid lowland zone around the area of the Belles River. The mean annual temperature varies between 25-400C with mean annual rain fall of 1569 mm. The livestock population of the district comprises about 70,403 cattle, 6,549 sheep, 24,995 goats, 1,232 equines, 30,997 poultry and 7,520 bee hives [25].
Study Animal
The study animal was indigenous zebu cattle reared extensively on communal grazing lands which were the principal feed source for cattle and other livestock during rainy season and crop residue were the major supplement available after harvest time. Only cattle’s aged above 1 year was considered and selected for the trial and allowed to graze together at the fringes of crop fields and fallow lands. They shared the same watering point during day time and housed at night on their respective farms. Animals obtain water in the rainy season from seasonal rivers while in the dry season from perennial rivers flowing in their locality.
Study Design
Both cross sectional and longitudinal study were conducted from February to June, 2021. The cross sectional study used to determine the prevalence of bovine trypanosomosis using microscopic/ parasitological and hematological examination while longitudinal study used to assess the level of trypanocidal drug resistance.
Sample Size Determination
To determine the prevalence of the disease in the Village (district sample size) a [26] formula was applied with an expected prevalence of 50%, 5% absolute desired precision and95% confidence level. Accordingly, sample Size was calculated:
N = (1.96)² Pexp (1-Pexp)/d²
Where: N = required sample size
Pexp = expected prevalence
d = desired absolute precision
A total of 384 cattle should have been needed to determine the prevalence of the disease as well as the PCV value of parasitic and a parasitic animals. However, to increase the chance of getting much number of positive animals which were subjected for drug susceptibility trial a total of 464 cattle’s were examined using a simple random sampling technique, where; 126, 182, 84, 72 animals were sampled from each village of Kuray, Achare, Addisalem and G/Wuha. The four hot spot villages were purposively selected from Jawi district as the areas were located in an ideal habitat for tsetse fly breeding. Hence, 25 trypanosome free animals were selected as a negative control and of 53 positive animals screened using a Buffy coat technique, 27 was grouped as a treatment group for Diminazene Aceturate (DA) and 26 for Isometamidium Chloride (ISM) treatment group. The four study sites were categorized into two (Achari and Kurey) for the purpose of effective monitoring.
Drug Susceptibility Study
Of 53 positive animals screened using a Buffy coat technique (Woo, 1970), 27 was grouped as a treatment group for Diminazene Aceturate (DA), 26 for Isometamidium Chloride (ISM). Placebo animals were used to guide for the reliability and disclose for the occurrence of any bias along the study period. Immediately to the selection, all animals under investigation were ear tagged based on their group of study and subjected to a deltamethrin 1% (Smash) pour-on, with 1ml/10kg bw (according to the manufacturer’s recommendation) along the back para-midline of the animal from shoulder to the base of the tail using T-bar applicator. This is important to expel fly contacts which are responsible to cause a bias on the study as the trial was conducted on extensively reared cattle’s. Before the actual treatment begins the animals were re-tested at day 0 to exclude for the presence of any latent trypanosome parasites infected animal from negative control groups and to realize the consistency of the parasites in positive animal groups. Indeed, of the 53 positive animals one was died before the actual treatment begun from ISM treatment group and the group was left with 25 animals. All the required parameters were recorded before and after the treatment; sex (male or female), body condition score (cachectic, lean and good [27], age group (<2 years, 2–5 years, and >5 years), coat color (red, black, white, gray or spoty), PCV (anemic <24% and non- anemic>24%) and species of trypanosome detected in the buffy coat (T. Conglense, T. vivax or mix and T. telerie).
A 28 day field protocol was used to estimate resistance to 3.5 mg/ kg/body weight diminazine aceturate and to 0.5 mg/kg/body weight isometamidium chloride in trypanosome positive cattle as described by [21]. Animals were randomly allocated to Diminazene (DIM) and Isometamidium (ISM) treatment groups. The animal’s weights were estimate during a weighing band as described by [27] to determine the dose of the drugs to be administered. All animals in group DIM received 3.5 mg/kg body weight of 7% solution Diminazine aceturate (DIMINAT®, Hebei Huarun, China) whereas group ISM was treated with 0.5mg/kg body weight of 1% solution Isometamidium chloride (ISM) (TRYPASHISH®, Tarapur, India). The drugs were administered intramuscular in gluteal and trapezium muscle for DIM and ISM respectively by veterinarians wherethis day was considered as day 0. Treated cattle were monitored for trypanosomes and PCV on days 14 and 28 post-treatment using parasitological and hematological technique. However, DIM treatment response was considered only at day 14 post treatment due to its short curative activity [28] and any animals that remain positive was treated with double dose of 7mg/kg body weight and followed for the extra 14 days. At the end of experimental trial all positive animals was treated/cleared with double dose of DIM 7mg/kg body weight.
Parasitological Examination and PCV Determination
The micro haematocrite capillary tubes were filled with the blood sample and sealed at one end using cristaseal. The capillary tubes were centrifuged at 12,000 Revolutions Per Minute (rpm) for 5 minutes to concentrate the trypanosomes in the Buffy coat layer [22]. First, the Packed Cell Volume (PCV) was measured soon after centrifugation using the Hawksley microhaematocrit reader (Hawksley, Lancing, United Kingdom). Then the capillary tube was placed in a Woo viewing chamber with a cover slip of 24 mm×24 mm and the Buffycoat plasma junction was examined under the microscope for the presence of trypanosomes. Trypanosome species were identified based on their movement using x 40 objective lens and ×10 eye pieces. Animals with PCV less than 24% considered to be anemic [29].
Ethical Consideration
Consent obtained from cattle owners and the livestock Office of the Woreda. Cattles used as control group during efficacy trial were disclosed for the owner for its negativity from trypanosome parasite at the end of the study. All animals involved in this study were handled according to standard guidelines for the use and handling of animals in research.
Data Management and Analysis
All data collected from parasitological and hematological investigation, questionnaire survey and trypanocidal drug resistance trial result was entered into Microsoft excel software making the data ready for further advanced software analysis (STATA, version 13 Stata Corp College Station, Texas, USA). Descriptive statistical analysis was done to assess the parasitological and hematological findings. An independent t-test and paired t-test was used to compare the parasite clearance ability of the drugs and the mean PCV value of animals under trial. A chi-square was also used to determine the association of covariates with response variables for the prevalence study. A p-value of <0.05 was considered as statistically significant across the study.
Results
Total Prevalence of Bovine Trypanosomosis in the Study Area
Of the 464 cattle that were examined in the 4 villages, 53 (11.4%) were trypanosome-positive (Table 1). The distribution of the disease across the villages were nearly similar with no significant difference (p=0.556). Among trypanosome-positive animals, 15% (8/53) were mixed infection for T. congelense and T. vivax and 7.5% (4/53) were T. telerie which is a large parasite in the field. Mean PCV in the studied cattle was 23.7% (95% CI: 23.3-24.1%) with almost no variation among the villages (Table 2). However, positivity for the parasite among anemic and non-anemic animals had a momentous variation (p=0.001) where anemic animals were more positive for the infection (41/258, 15.9%) than non-anemic animals (12/206, 5.8%) (Table 3). Among the trypanosome-positive cattle, those infected with T. congolense had relatively lower mean PCV value (18.5%) compared to the cattle infected with T.vivax (20.2%), mixed infection (18.8%) and T.telerie (21.5%) as showed in (Table 1).
Village
Trypanosome positive cattle
No. of cattle examined
Prevalence (95%CI)
Mean PCV (95%CI)
Tc
Tv
Tc&Tv (mix)
Tt
Total
Achare
6
3
7
1
17
182
9.3(5.8-14.6)
23.3(22.4-24.3)
Addisalem
4
9
-
-
13
84
15.5(9.1-25.1)
25.2(24.2-26.1)
G/Wuha
7
3
1
-
11
72
15.3(8.5-25.5)
22.1(21.0-23.2)
Kuray
6
3
-
3
12
126
9.5(5.4-16.1)
24.3(22.9-25.6)
Total
23
18
8
4
53
464
11.4(8.8-14.7)
23.7(23.3-24.1)
Table 1: Trypanosome prevalence and associated factors in bovines in the study area.
Variables
Total No. of samples
Positive samples
χ2
p-value
Village
2.0817
0.556
Achare
182
17 (9.3%)
Addisalem
84
11(13.1%)
Gingero Wuha
72
11(15.3%)
Kurey
126
14(11.1%)
Table 2: Trypanosome prevalence with respect to study site.
Variables
Total No. of samples
Positive samples
χ2
p-value
Age
1.0463
0.593
<=2years
74
11(14.9%)
2-5years
180
19(10.6%)
>5years
210
23(20.9%)
Sex
1.1826
0.277
Female
260
26 (10%)
Male
204
27(13.2%)
BCS
0.0106
0.918
Poor
422
48(11.4%)
Medium
42
5(11.9%)
Color
2.7686
0.736
Black
67
6 (8.9%)
Gray
59
8 (13.6%)
Pale red
14
1(7.1%)
Red
138
20(14.5%)
Spotted
103
10 (9.7%)
White
83
8(9.6%)
PCV
11.4715
0.001
<=24
258
41(15.9%)
>24
206
12(5.8%)
Table 3: Trypanosome prevalence withrespect to host related factor.
Village
Total trypanosome species under investigation
Total trypanosome parasite in each village
Negative controls
T.congelense
T.vivax
T.c&T.v (mix)
T.telerie
Achari
13
6
8
1
28
13
Kurey
9
12
0
3
24
12
Total
22
18
8
4
52
25
Table 4: Total cattle’s under trypanosome drug resistant trial in the study area.
Trypanocidal Drug Resistance Study
Parasitological result for the trail with respect to village and trypanosome species: Trypanosoma congolense was the dominant trypanosome species and accounted for 43.4% (23/53) of the entire trypanosome infections (Figure 4).
Parasitological responses to drug treatments:
Diminazene aceturate (DIM) treatment response: Trypanosome response to Diminazene aceturate (DIM) treatment relatively varied across the study villages (Table 5). Of the 27 trypanosome positive cattle treated with 3.5 mg/kg body weight DIM at day 0, 48.1% (13/27) had persistent trypanosomes 14 days post treatment. Achari village had the highest DIM treatment failures (50%) compared to Kurey village (44.4%). Trypanosoma congolense accounted 52.6% DIM treatment failure when compared to trypanosome vivax (20%). Although, only one animal infected for T. telerie had been investigated under DIM treatment, the drug failed to clear the parasite. Out of the five mixed infection for T. congolense and T. vivax, four T.congolense were failed to respond for the DIM and all T.vivax were cleared out. This signifies that, comparatively, T. vivax strains were apparently sensitive to 3.5 mg/kg DIM than T.congolense and T.telerie. Retreatment of trypanosome positive cattle’s which had failed DIM at 3.5mg/kg were subjected to double DIM dose (7 mg/kg DIM) to assess further drug response. Indeed, the result revealed a treatment failure of 53.8% (7/13) with the highest number of T.congolense positive as shown in (Table 5) and illustrated in (Figure 1).
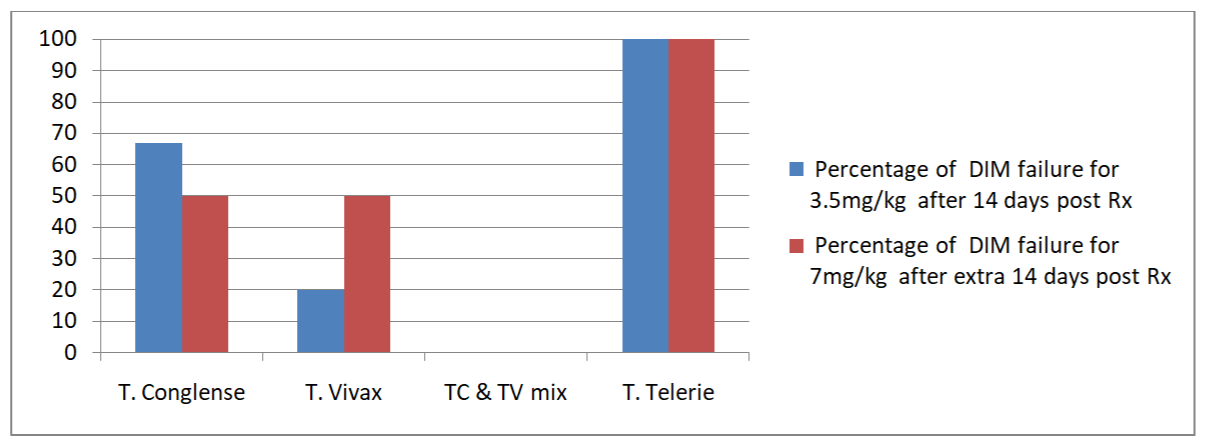
Figure 1: Status for DIM resistance among the parasites.
Villages
Failed treatment response 14 days post-treatment with 3.5 mg/kg bbw.
Failed retreatment response 14 days4 post-treatment with 7.0 mg/kg bw.
Tc1
Tv2
Tc&Tv (mix)
Tt3
Total
Tc1
Tv2
aMix
Tt3
Total
Achari
8/10
1/3
0/1
-
9/14(50%)
4/8
1/1
-
-
5/9(55.6%)
Kurey
2/5
1/7
-
1/1
4/13(44.4%)
1/2
0/1
-
1/1
2/4(50%)
Total
10/15
2/10
0/1
1/1
13/27(48.1%)
5/10
1/2
-
1/1(100%)
7/13(53.8%)
1Trypanosoma congolense; 2Trypanosoma vivax; 3Trypanosoma telerie; 4treatment response observed extra 14 days upon the first treatment status; aTc & Tv infection concurrently detected in the same animal; bbody weight.
Table 5: Cattle’s with failed DIM treatment over total treated in each village.
Isometamidium chloride (ISM) treatment response: At day 14 ISM post-treatment, trypanosome susceptibility to 0.5 mg/kg body weight showed some variability across trypanosome parasites (Table 5). Of the 25 trypanosome-positive cattle treated at this dose, 40% (10/25) still had persistent infections at the end of 28 days. However, upon a 14 day assessment for the status of the parasite 48% (12/25) were failed to respond for the drug. High level of ISM treatment failure was observed in Achari village 50% (7/14) as compared to Kurey 45.5% (5/11) on the early examination (i.e. 14 days post treatment). Among the ISM treated cattle, T. congolense accounted for about 62% (9/13) of the failed treatments. Trypanosoma vivax positive cattle that received ISM treatment in both villages were all cleared of this parasite. Of the 12 cattle aparasitaemic at day 14 post-treatment, 83.3% (10/12) were trypanosome-positive on day 28. Trypanosoma congolense accounted for 77.8% (7/9) of the failed treatments and all T.telerie that were positive at day 0 were not cleared out at both 14 and 28 days of post treatment. The cumulative treatment failure rate (summing days 14 and 28 failed ISM treatments) was 59.5% (22/37). Trypanosoma telerie and trypanosoma congolense accounted for 100% (3/3) and 71.4% (5/7) of all ISM treatment failures respectively as indicated in (Figure 2). Based on geographical location, Kurey village had relatively lower treatment failures (56.2%) compared to Achari village (61.9%) as indicated in (Table 6).
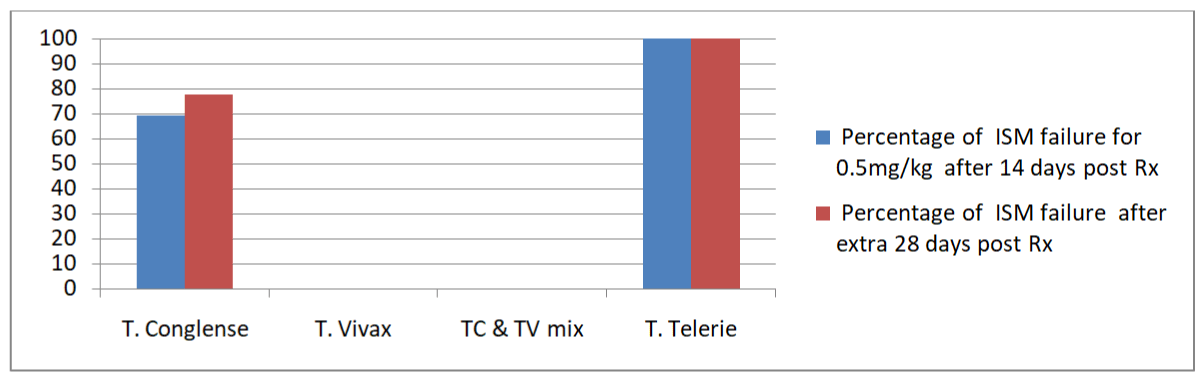
Figure 2: ISM treated trypanosome infected cattle’s drug failure status.
Villages
Failed treatment response 14 days post-treatment.
Failed retreatment response 28 days post-treatment.
Cumulative failed treatment response
Tc1
Tv2
aMix
Tt3
Total
Tc1
Tv2
aMix
Tt3
Total
Achari
6/9
0/3
0/1
1/1
7/14(50%)
5/6
-
-
1/1
6/7(85.7%)
13/21(61.9%)
Kurey
3/4
0/5
-
2/2
5/11(45.5%)
2/3
-
-
2/2
4/5(80%)
9/16 (56.2%)
Total
9/13
0/8
0/1
3/3
12/25(48%)
7/9
-
-
3/3
10/12(83.3%)
22/37(59.5%)
1Trypanosoma congolense; 2Trypanosoma vivax; 3Trypanosoma telerie; 4treatment response observed extra 14 days upon the first treatment status; aTc & Tv infection concurrently detected in the same animal.
Table 6: Cattle’s with failed Isometamidium Chloride (ISM) treatment over total treated in each village.
Packed cell volume of treated animals:
Response of PCV level among treatment and control groups: Treatment of the trypanosome-positive cattle with DIM significantly (p < 0.001) increased the PCV% from 19.9 % at day 0 to 21.2 % at day 14 and eventually to 23.8% at day 28. Likewise, ISM treated cattle’s were similarly improved the mean PCV value from 21.5% at day 0 to 23.9% at day 14 and in due course to 25.0% with a significant difference (p<0.001). However, the negative control for the PCV change have no significant variation (p=0.662) among the various days under investigation as illustrated in (Table 7). Based on trypanosome species, PCV% for those animals treated with DIM have a significant change over T.congolense (p=0.004) and T.vivax (p=0.004). Cattle with mixed infection (T.congolense and T.vivax) and T.telerie also showed an increment of PCV% from day 0 to the consecutive 14 and 28 days although the difference was not statistically significant as shown in (Table 8). As indicated in (Table 9), ISM treated cattle’s have showed a significant PCV% variation (p<0.001) for T. congolense from day 0 (20.8%) to 23.7% at day 14 and 24.7% at day 28. Similarly, T. telerie have showed a significant change (p=0.020) for PCV value over the consecutive follow up periods. In other words, Mixed (T. congolense and T. vivax) and T. vivax infections were none significantly (p>0.05) increased the PCV% along the track as point out in (Table 9). In general, when the status of PCV percentage compared for control and treatment groups, positive animals treated with drugs were relatively increased the PCV value than control group as indicated in (Figure 3).
Figure 3: Effect of PCV percentage among trypanosome parasites treated with DIM and ISM.
Day of treatment
DIM PCV% (95%CI)
ISM PCV% (95%CI)
Negative control (95%CI)
Day 0
19.9(18.3-21.6)
21.5(19.6-23.3)
28.1(26.4-29.8)
Day 14
21.2(19.3-23.0)
23.9(22.2-25.5)
27.8(26.7-29.0)
Day 28
23.8(22.4-26.3)
25.0(23.7-26.3)
28.2(27.0-29.4)
p-value: Day 0 &14
0.061
0.003
0.781
p-value: Day 14&28
<0.001
0.058
0.937
p-value: Day 0 &28
<0.001
<0.001
0.662
Table 7: Over all mean PCV% value among the treatment and control groups.
Day of treatment
Trypanosome species with respective PCV values
T.conglense
T.vivax
Tc & Tv (mix)
T.telerie
Day 0
20.1(18.1-22.1)
21.9(20.0-23.8)
19.4(14.9-23.8)
21.5(15.3-27.7)
Day 14
22.2(20.2-24.3)
23.1(21.0-25.2)
21.3(17.0-25.5)
24.3(16.7-31.8)
Day 28
23.7(22.2 25.1)
25.4(24.0-26.8)
22.8(19.2-26.3)
26.3(21.0-31.5)
p-value: Day 0 &14
0.125
0.394
0.483
0.402
p-value: Day 14&28
0.233
0.067
0.532
0.111
p-value: Day 0 &28
0.004
0.004
0.182
0.513
Table 8: Change in mean PCV values with (95%CI) for DIM treatment group.
Day of treatment
Trypanosome species with respective PCV values
T.conglense
T.vivax
Tc & Tv (mix)
T.telerie
Day 0
20.8(17.8-27.8)
23(19.4-26.6)
19.3(4.2-34.5)
23(15.5-30.4)
Day 14
23.7(20.9-26.5)
24.3(21.0-27.5)
22.7(13.9-31.4)
25.7(14.2-37.1)
Day 28
24.7(22.7-25.1)
25.1(22.4-27.8)
23(14.4-31.6)
27(18.0-36.0)
p-value: Day 0 &14
0.016
0.417
0.362
0.208
p-value: Day 14&28
0.305
0.371
0.742
0.547
p-value: Day 0 &28
<0.001
0.242
0.427
0.020
Table 9: Change in mean PCV values with (95% CI) for ISM treatment group.
Discussion
African animal trypanosomiasis (Nagana) is a group of protozoan parasitic diseases of ruminants, camels, equines, swine and carnivores caused by different trypanosome species. The major pathogenic species in African cattle are T. congolense, T. vivax, and, to a lesser extent, T. brucei [30]. Although hosts acquire infection principally via the bite of infected tsetse flies, other haematophagous insects like Tabanids and Stomoxys species also transmit trypanosomosis mechanically [31]. In domestic animals, trypanosomiasis is a disease with a great economic impact, affecting not only the well-being of the livestock population, but also efficient food production in croplivestock production systems [32]. Among all African pathogenic trypanosome species, Trypanosome congolense is arguably the one causing major losses in Sub-Saharan Africa [33].
Parasitological findings in the present study showed that the overall prevalence of trypanosome species at Jawi district was 11.4% (53/464). This finding coincides with previous reports by [23] who found a prevalence of 11.3% in the same district [34] also reported nearly similar prevalence (12.42%) in Metekel and Awi Zones which might be due to similarity in agro-ecological situation. The present survey revealed that T. congolense was the dominant species (15%, 8/53) found in the area compared to T.vivax (7.5%, 4/53). This result is in line with their ports by [35-38] in tsetse infested areas of Ethiopia.
Most trypanosome infected animal show anemia [37,35,38,39] due to destruction of red blood cell that could result in a drop of their PCV, hemoglobin and RBC count [40]. In concur with these reports, the current study also showed significantly reduced PCV in trypanosome positive animals compared to aparasitaemic animals. Similarly, positivity was more pronounced among anemic compared to non-anemic animals. Among the trypanosome-positive cattle, those infected with T. congolense had relatively lower mean PCV value as compared to cattle infected with T.vivax, mixed infection and T.telerie. This is due to the higher impact of T. congolense in the blood cell as compared to T.vivax which mostly invades tissues [41]. Although the size of T. telerie parasite was much larger under the field than the rest of the trypanosome species, its distribution was very limited where only one parasite was observed in the current study. Indeed, its ability in causing anemia was restricted as compared to other trypanosome species which were observed with considerable distribution. Hence, the development of anemia was mainly caused by T. congolense in the study area.
The overall parasitological failure of Diminazene aceturate at dose of 3.5mg/Kg bw, for 27 trypanosome positive animal at day 14 was 48.1% (13/27). Among DIM treated species T. congolense faced 52.6% of treatment failure as compared to T.vivax (20%) and one animal harbor in trypanosoma telerie was failed to be cleared by the drug. Indeed, the result revealed a treatment failure rate of 53.8% (7/13) with the highest number of T.congolense positive. In support of the current study different author show DIM resistance especially on T.congolense species in various area of Ethiopia, Ghibe valley, Metekel district, Upper Dedesa, Bedelle and Sodo and Arbaminch; conducted by [9,10,42,43]. Later on research conducted by [13], using DpnII-PCR-RFLP in the Ghibe valley also showed resistant strain of T.congolense species. The problem of drug resistance among T.congolense was speculated because of T.congolense confirmation mainly in the blood while T.vivax can also invade tissues [44,45].
With regard to ISM treatment group, parasitological response at day 14 with the applied dose level of 0.5 mg/kg body weight revealed 48% (12/25) were failed to respond for trypanosome parasites. About 62% (9/13) of T. congolense were failed to ISM treatment and all T. vivax species in both villages were cleared at the trial dose level. Out of 12 parasitemic animals at day 14 post-treatment 83.3% (10/12) were trypanosome-positive on day 28. About 77.8% (7/9) of Trypanosoma congolense acquired treatment failure. Similarly, all Trypanosoma telerie that were positive at day 0 were not cleared at day 14 and 28 post treatment. This might be due to the large size of T.thelerie compared to other small sized parasites (i.e.T.congolense and T.vivax) on the recommended dose level. In addition, the variation in morphological arrangement of the kinetoplast of the parasite affects the mode of action for Isometamidium Chloride (ISM); where its cleavage site was at kDNA topoisomerase complexes, causing the desegregation of the minicircle network within the kinetoplast [46]. However [47] showed that dyskinetoplastic trypanosomesare at least as sensitive to isometamidium as kinetoplastic lines. The cumulative treatment failure rate (days 14 and 28) for ISM was 59.5% (22/37). Of which Trypanosoma telerie and trypanosoma congolense accounted for 100% (3/3) and 71.4% (5/7) treatment failure respectively. These results are in accordance with earlier reports from [48,8] in Northwest Ethiopia and Ghibe valley respectively.
A significant (p < 0.001) hematological response was observed after animals were treated with DIM which increased the PCV value from 19.9 % at day 0 to 21.2 % at day 14 and 23.8% at day 28. This might be attributed to the effect of the drug at least in reducing the infection rate of the parasite in the red blood cells even though in most parasite species a considerably resistance against the recommended therapy have been observed. This is in accordance with a research conducted by [48] in tsetse and non-tsetse infested area of Northwest Ethiopia. Similarly, animals subjected to ISM group were also significantly improved (p<0.001) mean PCV value from 21.5% at day 0 to 23.9% at day 14 and 25.0% at day 28. However, no significant variation (p=0.662) was recorded among PCV value of the negative control animals. This clearly signifies that there was an impact that weakens the action of the parasite by limiting the reproduction capability in the host. Association of anemia with that of trypanosome infection was multifactorial. There was a slight PCV recovery after treatment with trypanocidal drugs [49]. This might be due to the reduced response of the bone marrow due to exhaustion when the infection runs a chronic course cannot be ruled out [50].
Conclusion and Recommendation
Trypanosomosis is the major parasitic diseases of livestock in Ethiopia. Trypanocidal drug were the principal method of disease control for animal Trypanosomosis but because of growing concern for drug resistance due to a wide therapeutic application of this drugs for parasitic control, the present study attempted to evaluate the situation of cattle Trypanosomosis and Trypanosomosis drug efficacy in purposively selected hot spot areas of northwestern Ethiopia. The causative parasite was prevalent with different magnitude within the study districts and the study findings divulgea high prevalence of the disease in the area with a dominant T.congolense parasite. Due to the effect of this dominant parasite, there was a significant drop in packed cell volume for those animal infected with this parasite. In general, this study showed the currently available and used trypanocidal drugs, particularly ISM and DIM were extensively failed to cure naturally trypanosome infected animals; and needs further investigations in other trypanosome prevalent areas to make evidence based decisions.
Based on the above conclusion the following recommendations are forwarded;
• There should be an awareness creation to vet professional, community animal health workers, and local administration bodies and livestock owners on storage, handling and application of trypanocidal drugs.
• There should have effective implementation of regulatory and legislative frame works quality of veterinary trypanocidal drugs, services coverage on veterinary and regular monitoring of trypanocidal drug efficacy.
• More recommended to control cyclical transmitting vectors using various control and eradication techniques due to its feasibility than chemotherapy application.
• A new trypanocidal drug discovery requires large amount of investment in terms of time, resources and human power so that it is recommended that drug application for trypanosome should be applied at appropriate time for appropriate case by appropriate person using appropriate drug type and regimen.
• Further investigation using sophisticated methods have been needed to identify the mechanism of resistance occurrence among these Heamoparasite.
Acknowledgements
The authors would like to thank National Animal Health Diagnostic and Investigation Center, for funding, logistic and technical supports of this research work and secondly the authors would like acknowledge Finote selam tsetse and Trypanosomosis control center and Bahir Dar Animal Health Investigation and Diagnostic Laboratory for their logistic and technical supports.
Conflict of Interest
The authors declare that they have no competing interests and have no any financial or personal relationships that could inappropriately influence or bias the content of the paper.
References
- Malone RW, Felgner PL, Verma IM. Cationic liposome-mediated RNA transfection. Proceedings of the National Academy of Sciences of the United States of America. 1989; 86: 6077-6081.
- Borji H, Naghibi A, Nasiri MR, Ahmadi A. Identification of Dactylogyrus spp. and other parasites of common carp in northeast of Iran. Journal of Parasitic Diseases. 2012; 36: 234-238.
- Courtin F, Jamonneau V, Duvallet G, Garcia A, Coulibaly B, Doumenge JP, et al. Sleeping sickness in West Africa (1906–2006): changes in spatial repartition and lessons from the past. Tropical Medicine & International Health. 2008; 13: 334-344.
- Geerts S, Holmes PH, Eisler MC, Diall O. African bovine trypanosomiasis: the problem of drug resistance. Trends in parasitology. 2001; 17: 25-28.
- Delespaux V, Geysen D, Bossche PVD, Geerts S. Molecular tools for the rapid detection of drug resistance in animal trypanosomes. Trends in parasitology. 2008; 24: 236-242.
- Chitanga S, Marcotty T, Namangala B, Bossche PVD, Abbeele JVD, Delespaux V. High Prevalence of Drug Resistance in Animal Trypanosomes without a History of Drug Exposure. PLoS Neglected Tropical Diseases. 2011; 5: e1454.
- Glover L, Hutchinson S, Alsford S, McCulloch R, Field MC, Horn D. Antigenic variation in African trypanosomes: the importance of chromosomal and nuclear context in VSG expression control. Cellular Microbiology. 2013; 15: 1984-1993.
- Codjia V, Mulatu W, Majiwa PA, Leak SG, Rowlands GJ, Authié E, et al. Epidemiology of bovine trypanosomiasis in the Ghibe valley, southwest Ethiopia. 3. Occurrence of populations of Trypanosoma congolense resistant to diminazene, isometamidium and homidium. Acta tropica. 1993; 53: 151- 163.
- Mulugeta W, Wilkes J, Mulatu W, Majiwa PA, Masake R, Peregrine AS. Long-term occurrence of Trypanosoma congolense resistant to diminazene, isometamidium and homidium in cattle at Ghibe, Ethiopia. Acta tropica. 1997; 64: 205-217.
- Afework Y, Clausen PH, Abebe G, Tilahun G. Mehlitz D. Multiple-drug resistant Trypanosoma congolensepopulations in village cattle of Metekel District, North WestEthiopia. Acta Trop. 2000; 76: 231-238.
- Tewelde N, Abebe G, Eisler M, McDermott J, Greiner M, Afework Y, et al. Application of field methods to assess isometamidium resistance of trypanosomes in cattle in western Ethiopia. Acta tropica. 2004; 90: 163-170.
- Shimelis D, Sangwan AK, Getachew A. Assessement of trypanocidal drug resistancein cattle of the Abay (Blue Nile) basin areas of North West Ethiopia. Ethiop Vet J. 2008; 12: 45-59.
- Moti Y, Fikru R, Abbeele JVD, Büscher P, Bossche PVD, Duchateau L, et al. Ghibe river basin in Ethiopia: present situation of trypanocidal drug resistance in Trypanosoma congolense using tests in mice and PCR-RFLP. Veterinary parasitology. 2012; 189: 197-203.
- Dagnachew S, Terefe G, Abebe G, Barry D, McCulloch R, Goddeeris B. In vivo experimental drug resistance study in Trypanosoma vivax isolates from tsetse infested and non-tsetse infested areas of Northwest Ethiopia. Acta tropica. 2015; 146: 95-100.
- Griffin L. An improved parasitological technique for the diagnosis of African trypanosomiasis?. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1978; 72: 212.
- Eisler MC, McDermott JJ, Mdachi R, Murilla GA, Shinyangwe L, Mubanga J, et al. Rapid method for the assessment of trypanocidal drug resistance in the field. In: Proceedings of the 9th symposium of the International Society for Veterinary Epidemiology and Economics (ISVEE) held in Breckenridge, Colorado, USA, August 6th – 11th 2000. 2000; (353): 1-3.
- McDermott J, Woitag T, Sidibé I, Bauer B, Diarra B, Ouédraogo D, et al. Field studies of drug-resistant cattle trypanosomes in Kénédougou Province, Burkina Faso. Acta tropica. 2003; 86: 93-103.
- Sinyangwe L, Delespaux V, Brandt J, Geerts S, Mubanga J, Machila N, et al. Trypanocidal drug resistance in eastern province of Zambia. Veterinary parasitology. 2004; 119: 125-135.
- Grace D. The epidemiology and control of trypanosomosis in areas of high risk of trypanocides resistance in West Africa. PhD Thesis, the Freie Universität Berlin. 2005: 195.
- Allegye-Cudjoe E. Field detection and evaluation of trypanocidal drug resistance in Ghana and Benin. MSc. dissertation, University of Edinburgh, UK. 2009; 60.
- Diall O, Clausen PH, Boucoum Z, Djiteye A, Diarra B, Grace D, et al. UN test de diagnostic rapide pour la détection et la surveillance sur le terrain dela résistance aux trypanocidal dans les zones cotonnières de l‟Afrique de l‟ouest. In:Proceedings of the 28th Meeting of the International Scientific Council forTrypanosomiasis Research and Control of the Organization of African Unity held inAddis Ababa, Ethiopia, 26th – 30th September. Publication 2005; 123: 367-375.
- Woo PTK. The haematocrit centrifuge technique for the diagnosis of Africantrypanosomiasis. Trop. Anim. Health Prod. 1970; 1: 89-95.
- Dagnachew S, Girma H, Abebe G. A cross-sectional study on bovine trypanosomosis in Jawi district of Amhara Region, northwest Ethiopia. Ethiopian Veterinary Journal. 2011; 15: 69-78.
- Yifat D, Ando O and Rahmato A. Trypanosoma Specie Causing Bovine Trypanosomosis in South Achefer District, Northwest Ethiopia. J. Vet. Adva. 2012. 2: 108-113.
- AZARD: Awi Administrative Zone Agricultural and Rural Development; Annual report. 2008.
- Thrustfield, M. Sampling. In: Veterinary epidemiology. 4th ed. Blackwell Science Ltd., UK. 2007; 221-254.
- Nicholson MJ and Butterworth MH.A Guide to Condition Scoring of Zebu Cattle. ILCA: Addis Ababa Ethiopia. 1986; 212–315.
- Mekonnen HM and biruk T. Heart girth-body weight relationship in two Ethiopian zebu breeds. Revue de Médecine Vétérinaire. Tome: 2004; 155: 512-515.
- Holmes PH, Eisler MC, Geerts S. Current chemotherapy of animal trypanosomiasis. The trypanosomiasis. Edited by: Maudlin I, Holmes PH, Miles MA. CABI International, Wallingford, UK, 2004; 431-444.
- OIE. Standardized techniques for the diagnosis of tsetse transmitted trypanosomiasis. in OIE Terrestrial Manual, 2008; 49. OIE, Rome, Italy.
- Taylor K, Authie E. Pathogenesis of Animal trypanosomiasis. In: Maulidn, I., Holmes, P., and Miles M. (ed.): The Trypanosomiases. UK: CABI International Walling ford. 2004: 331-353.
- Eisler M, Dwinger R, Majiwa D, Picozzi K. Diagnosis and Epidemiology of African Animal trypanosomiasis. In: Maudlin, I., Holmes, P., and Miles, M. (ed.): The Trypanosomiases. UK: CABI, CAB International. 2004; 253-268.
- Shaw A, Cecchi G, Wint G, Mattioli RC, Robinson TP. Mapping the economic benefits to livestock keepers from intervening against bovine trypanosomosis in Eastern Africa. Preventive veterinary medicine. 2014; 113: 197-210.
- Mekuria S, Gadissa F. Survey on bovine trypanosomosis and its vector in Metekel and Awi zones of Northwest Ethiopia. Acta tropica. 2011; 117: 146- 151.
- Dagnachew S, Sangwan AK, Abebe G. Epidemiology of Bovine Trypanosomosis in the Abay (Blue Nile) Basin Areas of Northwest Ethiopia. Revue d’élevage et de médecine vétérinaire des pays tropicaux. 2005; 58: 151.
- Fikru R, Goddeeris BM, Delespaux V, Moti Y, Tadesse A, Bekana M, et al. Widespread occurrence of Trypanosoma vivax in bovines of tsetse- as well as non-tsetse-infested regions of Ethiopia: a reason for concern?. Veterinary parasitology. 2012; 190: 355-361.
- Cherenet T, Sani RA, Speybroeck N, Panandam JM, Nadzr S, Bossche PVD. A comparative longitudinal study of bovine trypanosomiasis in tsetse-free and tsetse-infested zones of the Amhara Region, northwest Ethiopia. Veterinary parasitology. 2006; 140: 251-258.
- Sinshaw A, Abebe G, Desquesnes M, Yoni W. Biting Flies and Trypanosome vivax Infection in Three Highland Districts Bordering Lake Tana, Ethiopia. Veterinary Parasitology. 2006; 141: 35-46.
- Degu F, Ayalew B, Tewdros F, Mersha C. Occurrence of bovine trypanosomosis, in the Blue Nile river basin, Northwest Ethiopia. Europ J of Appl Scie. 20124; 4: 129-135
- Sumbria D, Singla LD, Sharma A, Moudgil AD, Bal MS. Equine trypanosomosis in central and western Punjab: prevalence, haemato-biochemical response and associated risk factors. Acta tropica. 2014;138:44-50. doi:10.1016/j. actatropica.2014.06.003
- Abebe G, Jobre Y. Trypanosomosis: A Threat to Cattle Production in Ethiopia. Revue de Medicine Veterinarie. 1996; 147: 897-902.
- Tewolde, N. Study on the occurrence of drug resistant trypanosomes in cattle in the farming in tsetse control areas (FITCA) project in Western Ethiopia. MSc thesis, Addis Ababa University, Faculty of veterinary medicine, Ethiopia. 2001.
- Chaka1 H, Abebe G. Drug Resistant Trypanosomes: a Threat to Cattle Production in the Southwest of Ethiopia. Revue Élev. Méd. vét. Pays trop. 2003; 56: 33-36
- Rowlands GS, Mulatu W, Authie E, Leak SG, Peregrine A.Epidemiology of Bovine Trypanosomosis in the Ghibe valley, Southwest Ethiopia. Acta Tropica. 1995; 53: 135-150.
- Stephen LE. Trypanosomiasis: a veterinary perspective. Pergamon Press, Oxford, UK. 1986; 551.
- Shapiro TA, Englund PT. Selective cleavage of kinetoplast DNA minicircles promoted by antitrypanosomal drugs. Proceedings of the National Academy of Sciences of the United States of America. 1990; 87: 950-954.
- Kaminsky R, Schmid C, Lun ZR. Susceptibility of dyskinetoplastic Trypanosoma evansi and T. equiperdum to isometamidium chloride. Parasitology Research. 1997; 83: 816-818.
- Dagnachew S, Tsegaye B, Awukew A, Tilahun M, Ashenafi H, Rowan T, et al. Prevalence of bovine trypanosomosis and assessment of trypanocidal drug resistance in tsetse infested and non-tsetse infested areas of Northwest Ethiopia. Parasite Epidemiology and Control. 2017; 2: 40-49.
- Holmes PH, Jennings FW. Pathogenicity of parasitic infections, Academic Press New York, ISBN 0372-5480, New York, USA. 1976.
- Murray M, Dexter TM. Anaemia in bovine African trypanosomiasis. A review. Acta tropica. 1988; 45: 389-432.