
Review Article
Austin J Vet Sci & Anim Husb. 2022; 9(4): 1102.
Diagnostic Techniques for Infectious Bovine Rhinotracheitis: A Review
Dima C¹* and Abdisa K²
1Animal Health Institute, Sebeta, Ethiopia
2Ambo University, College of Agriculture and Veterinary Science, Ambo, Ethiopia
*Corresponding author: Chala Dima, Animal Health Institute, P.O. Box: - 04, Sebeta, Ethiopia
Received: August 24, 2022; Accepted: September 15, 2022; Published: September 22, 2022
Abstract
Infectious Bovine Rhinotracheitis (IBR) is a group of the bovine respiratory disease multifaceted pathogens and a key disease of cattle, leading to significant economic losses to the dairy industry globally. The causative agent of IBR is Bovine herpesvirus type-1 (BoHV-1), which is a member of the genus Varicellovirus in the Alphaherpesvirinae subfamily, which belongs to the family Herpesviridae, order Herpesvirales. BoHV-1 can be categorized into three subtypes (BoHV-1.1, BoHV-1.2a, and BoHV-1.2b) that belong to one single viral species, which is a serologically indistinguishable strain. Therefore, a more optimal method for the rapid diagnosis of BoHV-1 infection is highly needed. Hence, the objective of this paper is to review the appropriate diagnostic techniques for the IBR virus in infected cattle. In this review, various rapid and confirmatory diagnostic methods used for the diagnosis of BoHV-1 infection were briefly described. BoHV-1 can be routinely detected by virus neutralization tests and enzyme-linked immunosorbent assays (indirect or blocking ELISA). IBRgEELISA is the most specific serological test for BoHV-1 and is recommended for marker vaccine to differentiate wild infection from vaccination schemes. Furthermore, virus isolation from tissue or swab samples by cell culture and DNA detection with LAMP, PCR, and real-time PCR techniques are all used to detect infected cattle. Direct sequencing of the entire genome using the Sanger sequencing method recently allowed for the differentiation of BoHV-1 subspecies and the distinction of the BoHV-1 field strain from vaccine strains based on single nucleotide polymorphisms. As the gold standard diagnosis for IBR is virus isolation in cell culture, commonly followed by BoHV-1 gene sequencing, it is also recommended.
Keywords: Bovine herpesvirus; Diagnostic techniques; ELISA; IBR; PCR; Virus neutralization
Introduction
Infectious Bovine Rhinotracheitis (IBR) is one of the bovine respiratory disease multifaceted pathogens, which is the major cause of cattle death around the world [1]. IBR is a key disease of cattle, causing considerable economic losses to the dairy industry globally [2,3]. BoHV-1 is a member of the genus Varicellovirus in the Alphaherpesvirinae subfamily which belongs to the family Herpesviridae, order Herpesvirales [4,5] and it is an etiological agent responsible for the development of a severe respiratory form of infection known as IBR in all cattle [6,7], and Infectious Pustular Vulvo-Vaginitis (IPV) and Infectious Pustular Balanoposthitis (IPB) in cows and bulls respectively [8].
Bovine herpesvirus type-1 is also one of the most essential viral infections of buffaloes globally [9], except in the BoHV-1 free countries [10]. BoHV-1 can be sub-grouped into three-subtypes belonging to one single viral species [11], in which they are serologically indistinguishable strains (BoHV-1.1, BoHV-1.2a, and BoHV-1.2b) [12]. Those animals infected with BoHV-1 experience a variety of mild to severe clinical syndromes, including rhinotracheitis, vaginitis, balanoposthitis, abortion, conjunctivitis, and enteritis, as well as decreased milk production and weight gain [2], and cough, nasal discharge, and lachrymal discharge with mild diarrhea [13].
Clinical indicators, species affected, epidemiological pattern, post-mortem lesions, and laboratory confirmation by isolation of the etiological agent utilizing different serological and molecular approaches can all be used to make a preliminary diagnosis of IBR. Virus Isolation (VI), Fluorescent Antibody technique (FAT), antigen detection by ELISA, and immunoperoxidase assays are being employed in diagnostic virology laboratories to identify BoHV-1. The indirect fluorescent antibody test (IFA) detects immunoglobulin M (IgM) and G (IgG) antibodies in serum in a semi-quantitative, sensitive, and rapid manner [14]. BoHV-1 abortion is diagnosed by the existence of appropriate histological lesions, VI, and Immunohistochemistry (IHC) for viral antigen identification. With sensitivity similar to that of IHC, BoHV-1 has been detected in tissues [15].
The in-vitro diagnosis of the disease based cell lines, on the other hand, is difficult to maintain, making the procedure inconvenient, time consuming, and expensive. IBR diagnostic procedures frequently employ VI in cell culture. The infectiousness of the viral particle is not required for enzyme immunoassays for antigen and antibody detection, but the results are harmed if the virus is destroyed. As a result, insufficient sample preservation and transit to the laboratory has a negative impact on the diagnosis [16]. The development of nucleic acid approaches for the identification of viruses in clinical material has received recent interest in diagnostic virology. Indirect Immunofluorescence tests (IFAT), Real-Time Polymerase Chain Reaction (RT-PCR), and nucleic acid hybridization are also often utilized for BoHV-1 diagnosis [17].
Although Polymerase Chain Reaction (PCR) in conjunction with southern blot hybridization has been shown to be better sensitive than VI and dot-blot hybridization screening, a large number of extended semen by these techniques is time-consuming. Because of its rapidity, sensitivity, and specificity, PCR has been developed as an ideal diagnostic tool for the detection of BoHV-1 in a variety of clinical samples [16]. As a result, efforts have been undertaken to standardize and verify a real-time PCR protocol [18] for detecting BoHV1 in naturally infected cattle and buffalo, as well as to use a sensitive, specific, and repeatable technique [19]. Also, a PCR technique was used to identify the BoHV-1 gB and gE genes in semen samples from naturally infected bulls, and the sensitivity was determined [8].
Generally in the context of animal health control and disease prevention, having access to accurate diagnostic tests having the ability of identifying early outbreaks and/or ensuring the nonexistence of the disease within vast territorial extensions is crucial [20]. As a result, early diagnosis of IBR virus-positive animals is critical for disease management and eradication efforts aimed at easing the burden on the dairy sector. Therefore, the goal of this paper is to review the appropriate diagnostic techniques recently in use for the diagnosis of infectious bovine rhinotracheitis virus in infected cattle.
Literature Review
Diagnostic Techniques for IBR
Currently, available diagnostic techniques are limited to the laboratory and require the use of sophisticated tools by specially trained staff members. To differentiate the BoHV-1 subtypes, numerous techniques have been utilized, including monoclonal antibody-specific antigen and DNA fingerprinting by recognizing the existence of the restriction site [21]. Bovine herpesvirus type-1 can be routinely diagnosed by cell culture, ELISA, virus neutralization tests, and molecular techniques by Polymerase Chain Reaction (PCR) [22].
Although in-vitro viral isolation in cell culture is still the gold standard for detecting BoHV-1, it has drawbacks in terms of sensitivity, sperm cytotoxicity, time-consuming, and expense. Several PCR approaches for detecting BoHV-1 have been shown to be effective [19]. The loop-mediated isothermal amplification (LAMP) assay has been proposed as a simple, quick, and alternative molecular pathogen diagnostic tool for field testing [23]. In order to differentiate BoHV-1 field strain from the vaccination strain based on Single Nucleotide Polymorphisms (SNPs), full genome sequencing was recently required [24,25].
Serological tests: The serological tests frequently used for the diagnosis of BoHV-1 antibodies in the serum samples of animals comprise the indirect ELISA, blocking ELISA, and the Virus Neutralization Test (VNT) [15]. Because of the short-time it takes to get a response, its convenience for screening large numbers of serum samples, and the best performance of any serological test used for IBR diagnosis, the indirect ELISA is utilized more often. Furthermore, because BoHV-1 viral latency is common, identifying serologically positive and otherwise healthy animals might be a good predictor of infection level in a herd. As a result, antibodiespositive animals must be categorized as BoHV-1 infected (with two exceptions: serological responses caused by inactivated vaccine immunization or colostral antibodies) [26]. Hence, IBRgE blocking ELISAs discriminate antibodies against the absent antigen, allowing infected and vaccinated animals to be distinguished (DIVA). Because the virus might reactivate during stress or sickness, blood should be drawn for antibody testing during the acute phase and again 2 - 4 weeks later [27].
A. Indirect ELISA: This diagnostic technique is used with coated antigens into the wells of a polystyrene plate. Antibodies bind to the coated antigen and are identified using enzyme-labeled antibovine immunoglobulins if present [28]. The number of antibodies in a sample can be represented in a variety of ways, but in general, the greater the Corrected Optical Density (COD), the more antibodies there are in the sample. Because of the backdrop of negative samples, cut-off numbers may vary, although they will be determined within each test [27] (as illustrated in Figure 1 below).
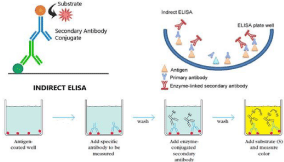
Figure 1: Schematic diagram of the Indirect ELISA test [27].
B. Blocking ELISA or C-ELISA: For DIVA purposes, this test was utilized in combination with the marker vaccinations. Marker-vaccinated animals exposed to a field strain of BoHV-1 may be differentiated from those that have not been exposed using enzyme-labeled monoclonal antibodies specific for gB or gE. Bovine herpesvirus type-1 antigen coated microwells on a polystyrene plate are used for C-ELISAs. The test serum samples were mixed with enzyme-labeled anti-BoHV-1 antibody and incubated. The quantity of BoHV-1 antibodies in the sample reduces the color development following the addition of the substrate/chromogen solution semiquantitatively. Cut-off values for negative tests should be below 0.5% and above 50% for positive results [27].
Virus Neutralization Test (VNT): The conventional VNT was employed to identify antibodies against BoHV-1 because of its excellent sensitivity and specificity. In two wells of tissue culture plates, 50μl of each serum sample and virus suspension were added and cultured for 2hrs at 37°C in the presence of 5% CO2. Then, the samples were cultured for 24–48hrs after adding 50μl of cell suspension (300,000 cells/ml) [29]. The neutralization index is calculated after incubation. The viral titer (in log10) in the existence of negative control serum minus the virus titer in the presence of particular antiserum is the neutralization index. The isolate may be classified as BoHV-1 if the neutralization index is more than 1.5, while conclusive confirmation would need molecular characterization to identify it from associated bovine alphaherpesviruses [30].
Agar gel precipitation test: The AGPT was carried out in accordance with [31]. In 100 ml of distilled water, 11/2 gm of agarose and 11/2 gm of glycine were added. In a water bath, the mixture was boiled until the agarose was mostly dissolved. The agarose medium was allowed at room temperature until it reached 45°C before being poured into 5 cm diameter petridishes. After the agarose in the petridishes solidified, 7 wells with a diameter of 6 mm were cut. Then the central well was filled with reference BoHV-1 homogenized collected pock lesions, whereas the upper and lower wells added serum containing positive and negative control sera. The four peripheral wells were filled with the sera samples to be evaluated and incubated for 12 to 72hrs at 37°C in a humidified atmosphere, looking for the formation of perception lines [28].
Indirect Immunofluorescence Assay (IFA): The IFA technique was carried out in tissue culture microtitration plates as a duplicate per sample according to [32] after some modifications. The MDBK cells were inoculated with positive culture supernatants, washed with PBS (pH 7.6), fixed for 30 minutes with fixation buffer, rewashed with PBS, and reacted with the reference rabbit anti-BoHV-1 polyclonal antiserum for an hour (diluted 1:100 in PBS). The MDBK cells were then rinsed in PBS (pH 7.6) and probed with anti-rabbit IgG that was fluorescence isothiocyanate-labeled (diluted to 1:50 in PBS). Microtitration plates were rinsed three times in PBS, mounted with 50% buffered glycerol, protected from light (kept in a dark place), and examined under a fluorescence microscope for greenish yellow fluorescence [33].
Virus Isolation by Cell Culture
Inoculated Madin-Darby Bovine Kidney (MDBK) cells were used to isolate the viruses from the positive samples. Samples were homogenized in complete Dubblicus Maintenance Eagle Medium (DMEM) and centrifuged at 12,000×g for 10 min at 4°C. The supernatants were then collected, passed through 0.22 μm membrane filters, and inoculated into MDBK cells for 2hrs, followed by the addition of virus growth medium containing 2% FBS and incubation at 37°C, with 5% CO2 [34]. After the third passage, if no CPE was observed, the sample was deemed negative. Inoculated MDBK cell culture with suspected BoHV-1 virus 107 TCID 50%, showed Cytopathic Effect (CPE) of cell rounding, cell aggregation, and clusters of rounded cell formation using Hematoxylin and Eosin staining, in which the peculiar cell changes were observed [35] as illustrated in Figure 2B above.
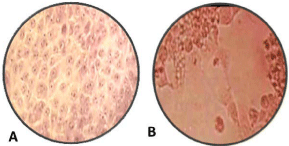
Figure 2A: Non-infected MDBK cell culture (control); B: Inoculated MDBK
cell culture (magnification power 100X) [36].
VantixTM Biosensor Assay
Biosensor-based assays are tools used to detect an antigen or antibody. In such assays, there is the use of receptors (antibodies) as well as transducers, which convert a biological interaction reaction into a quantifiable signal. The biosensors are attached to very sensitive instruments able to capture readable signals. These serum samples were diluted 1:10 in PBS (PH 7.2) with 2.5% gelatin from cold water fish skin immediately before testing, while milk samples were tested undiluted [37,38].
Individual biosensors are washed using a simple slotted cork strip into which the electrical connectors of up to 12 biosensors can be plugged. Samples were incubated for 5 minutes in a microtitre plate containing rabbit anti-bovine horseradish peroxidase conjugated secondary antibody (same as ELISA) diluted 1:20,000 in PBS (pH 7.2) and then washed. The wash/blot procedure was repeated four times in a new trough of wash buffer. Then, the electrical connector end of the biosensor was inserted into the Vantix™ Version 1 Research Reader prior to placing the electrodes into the TMB substrate and measuring the OD value [38].
Molecular Techniques
Polymerase Chain Reaction: A Polymerase Chain Reaction (PCR) has been utilized to directly detect BoHV-1 nucleic acid in specimens [39,41]. BoHV-1 and other herpesvirus DNA will be recovered from 200 μL of infected cell culture supernatant using the UNIQ-10 viral DNA extraction kit. The extracted DNA will be utilized as a template for copy DNA synthesis using the Revert AidTM First Strand cDNA Synthesis Kit, which will be eluted in 50 μL of nuclease-free water, and all these templates will be kept at -70 °C for later use [40]. BoHV-1 can be diagnosed using a simple real-time multiplex PCR (RT-PCR) [41], and it has been shown to be the most successful technique for testing for BoHV-1 in bovine abortions, even from autolyzed fetuses [42,43].
Loop Mediated Isothermal Amplification (LAMP) Assay: Several isothermal amplification tests for the detection of human and animal pathogen infections have recently been developed [44], enabling quick and specific diagnoses with little equipment [40,45]. LAMP is a novel assay that rapidly amplifies nucleic acids with high specificity under isothermal conditions [46,47]. The LAMP is initiated by an inner primer including sequences from the target DNA’s sense and antisense strands. A single-stranded DNA is produced by the following strand displacement DNA synthesis, which is primed by an outside primer. The end result is stem–loop DNA with many inverted loops, with 109 copies of the target accumulating in less than an hour. Bovine herpesvirus-1 LAMP assays were composed of 6μL of Isothermal Master Mix (Optigen ISO-001) and 2.5 μL of DNA sample [48,49]. Reactions were run in the thermoblock for 60 minutes at 66oC. Results were visualized by the addition of 1 μL of 1000x concentrated SYBR Green per sample [50]. Positive samples turned green, while negative samples remained orange, as indicated in Figure 3 above.

Figure 3: Visual detection of LAMP products using diluted SYBR Green stain
[49].
Real-time-PCR assay: The real-time PCR reaction was conducted in accordance with the OIE Manual [30], with minor modifications, in a Bio-Rad iCycler iQ. BoHV-1 was detected directly from swab samples and/or from a virus propagated in MDBK monolayer cell lines. Viral DNA will be extracted from each dilution using a Qia Blood mini kit, and the Cycle Threshold (Ct) value will be calculated. Any sample with a Ct value of <40 will be regarded as positive, while any sample with no Ct value at all will be considered negative [15].
BoHV-1 gB and TK direct sequencing: Polymerase chain reaction products were purified with the QIA quick purification kit (Qiagen) and sequenced with the Big Dye Terminator Cycle Sequencing Kit using PCR primers as sequencing primers. The sequencing of BoHV- 1, Thymidine Kinase (TK), and gB genes was performed according to the Sanger sequencing method and is one of the best performing IBR diagnostic techniques in recent times. In the cycle sequencing process, an initial denaturation at 96°C for 2 minutes is followed by 40 repeated cycles of denaturation at 96 °C for 10 seconds, annealing at 50 °C for 5 seconds, and elongation at 60°C for 4 minutes in the cycle sequencing process. Isopropanol was used to purify the products, which were then denatured at 95°C for 2 minutes. The ABI Prism 310 Genetic Analyzer (Applied Biosystems) was used to evaluate the sequencing processes, and the nucleotide sequences of the BoHV-1 TK and gB genes were aligned and compared with viral sequences in the GenBank database using BLAST software [51].
Conclusion and Recommendations
Infectious Bovine Rhinotracheitis (IBR) surveillance, diagnosis, and control depend on the clinical signs of the disease and accurate antibody detection of BoHV-1 from serum or milk samples using viral neutralization tests and ELISA techniques. The results obtained from serological investigation could be enough for the determination of the IBR status of individual animals and the population at large because of latent infection induced by BoHV-1 antibody detection. The development of nucleic acid approaches for the detection of viruses in clinical specimens has recently attracted interest in diagnostic virology. Furthermore, virus isolation and DNA detection from tissue or swab samples by cell culture, LAMP, and PCR techniques are all used to diagnose IBR. Direct sequencing of BoHV-1 genes according to the Sanger sequencing method provides a definitive diagnosis and is used to differentiate the three subspecies of BoHV-1. The gold standard diagnosis for IBR is virus isolation in cell culture, commonly followed by PCR analysis, and BoHV-1 gene sequencing is essential.
Based on the above conclusion, the following recommendation is forwarded. The IBR gE ELISA test is the most BoHV-1-specific serological test available, and it is recommended for marker vaccines (animals vaccinated for IBR disease) to differentiate wild infection from the vaccination scheme. The gold standard diagnosis for IBR is virus isolation in cell culture, commonly followed by PCR product analysis and BoHV-1 gene sequencing.
Acknowledgment
I would like to convey my deepest thanks to Dr. Kebede Abdisa for his informed counsel, kind and unwavering support in commenting and offering insights during the preparation of this paper.
Author Contributions
Both authors contributed significantly to the work presented. Participated in the article’s preparation, revision, or critical review; granted final approval of the published version; and agreed on the journal to which the article should be submitted.
Disclosure
In this work, the authors state that they have no competing interests.
References
- Kirchhoff J, Uhlenbruck S, Goris K, Keil G, Herrler G. Three viruses of the bovine respiratory disease complex apply different strategies to initiate infection. Vet Res. 2014; 45: 1-12.
- Raaperi K, Orro T, Viltrop A. Epidemiology and control of bovine herpesvirus-1 infection in Europe. Vet J. 2014; 201: 249–56.
- Newcomer B, Givens D. Diagnosis and control of viral diseases of reproductive importance: Infectious Bovine Rhinotracheitis and bovine viral diarrhea. Vet Clin North Am Food Anim Pract. 2016; 32: 425–441.
- Andrew J. Herpesvirus systematics. Vet Microbiol. 2010; 143: 52–69.
- OIE. Manual of diagnostic tests and vaccines for terrestrial animals: mammals, birds and bees, Office International des Epizooties, Paris, France. 2017: 1–15.
- Rola J, Larska M, Socha W, Rola G, Materniak M, Urban-Chmiel R. Seroprevalence of Bovine herpesvirus 1 related alpha herpesvirus infections in free-living and captive cervids in Poland. Vet. Microbiol. 2017; 204: 77–83.
- Thakur V, Kumar M, Rathish R. Seroprevalence of Bovine herpesvirus-1 antibodies in bovines in five districts of Uttarakhand. Vet. World. 2017; 10: 140–143.
- Keneisezo K, Neithono K, Keneisevono K, Limasenla P, Kevisenuo E, Sathiyabama k. Bovine herpes virus -1 (BoHV-1) in cattle- a review with emphasis on epidemiological parameters influencing the prevalence of bovine herpes virus -1 in cattle in India. J Entomol Zool Stud. 2019; 7: 284- 290.
- Woodbine K, Medley G, Moore S, Ramirez-Villaescusa A, Mason S. A fouryear longitudinal sero-epidemiological study of bovine herpesvirus type-1 (BoHV-1) in adult cattle in 107 unvaccinated herds in South West England. BMC Vet Res. 2009; 5: 5.
- Sharad R, Tiwori R. Complex scenario of Bovine herpesvirus-1 (BoHV-1) vaccines. J Antivir Antiretrovir. 2016; 8: 60-61.
- Mac-Lachlan N, Dubovi E. Fanner’s Veterinary Virology. London Acad.Press. 2011; 4: 180.
- Graham D. Bovine herpesvirus-1 (BoHV-1) in cattle - a review with emphasis on reproductive impacts and the emergence of infection in Ireland and the United Kingdom. Ir Vet J. 2013; 66: 15.
- Samrath D, Shakya S, Rawat N, Gilhare V, Singh F. Isolation and adaptation of bovine herpes virus Type 1 in embryonated chicken eggs and in Madin– Darby bovine kidney cell line. Vet World. 2016; 9: 222-225.
- Vira S, Mekhedov E, Humphrey G, Blank P. Fluorescent labeled antibodies - balancing functionality and degree of labeling. Anal. Biochem. 2010; 402: 146-50.
- Mahajan V, Banga H, Deka D, Filia G, Gupta A. Comparison of Diagnostic Tests for Diagnosis of Infectious Bovine Rhinotracheitis in Natural Cases of Bovine Abortion. J Comp Pathol. 2013; 149: 391-401.
- Majumder S, Ramakrishnan M, Nandi S. Infectious Bovine Rhinotracheitis: An Indian Perspective. Int J Curr Microbiol Appl Sci. 2015; 4: 844-858.
- Majumder S, Pandey A, Ramakrishnan M. Sero-epidemiology and detection of bovine herpesvirus 1 (BoHV1) antigen in semen of cattle and buffalo by polymerase chain reaction. Indian J. Vet. Pathol. 2013; 37: 118–120.
- OIE. Manual of diagnostic tests and vaccines for terrestrial animals, 6th Ed. OIE, Paris, 2008; 752-767.
- Kumar S, Leela N, Kota S, Narasimha P, Rajan S, Alwar V. Use of real-time polymerase chain reaction to detect bovine herpesvirus-1 in frozen cattle and buffalo semen in India. VeterinariaItaliana. 2011; 47: 313-322.
- Casarin E, Lucchese L, Grazioli S, Facchin S, Realdon N, Brocchi E, et al. A New ELISA Using the ANANAS Technology Showing High Sensitivity to diagnose the Bovine Rhinotracheitis from Individual Sera to Pooled Milk. PLoSONE. 2016; 11: 1-12.
- Vaz P, Horsington J, Hartley C, Browning G, Ficorilli N, Studdert M, et al. Evidence of widespread natural recombination among field isolates of equine herpesvirus-4 but not among field isolates of equine herpesvirus-1. J Gen Virol. 2016; 97: 747-755.
- Biswas S, Bandyopadhyay S, Dimri U, Patra H. Bovine herpesvirus-1 (BoHV- 1) a re-emerging concern in livestock: A revisit to its biology, epidemiology, diagnosis, and prophylaxis. Vet Q. 2013; 33: 68-81.
- Suwancharoen D, Sittiwicheanwong B, Wiratsudakul A. Evaluation of loop mediated isothermal amplification method (LAMP) for pathogenic Leptospira spp. detection with Leptospira isolation and real-time PCR. J Vet Med Sci. 2016; 78: 1299–1302.
- Fulton R, d’Offay J, Eberle R, Moeller R, Van Campen H, et al. Bovine herpesvirus-1: Evaluation of genetic diversity of subtypes derived from field strains of varied clinical syndromes and their relationship to vaccine strains. Vaccine. 2015; 33: 549-558.
- Fulton R, d’Offay J, Dubovi E, Eberle R. Bovine herpesvirus-1: Genetic diversity of field strains from cattle with respiratory disease, genital, fetal disease and systemic neonatal disease and their relationship to vaccine strains. Virus Res. 2016; 223: 115-121.
- Chatterjee A, Bakshi S, Sarkar S, Mitra J, Chowdhury S. Bovine herpes virus-1 and its infection in India - a review. Indian J Anim Hlth. 2016; 55: 21-40.
- Peter N, George R. Update on infectious bovine rhinotracheitis. Group BMJ Com. 2017; 39: 255-272.
- Zeedan G, Abdalhamed A, Ghazy A, Ghoneim N. Serological and Molecular Identification of Infectious Bovine Rhinotracheitis Virus Isolation and Adaptation in Embryonated Chicken Eggs. J Anti vir Antiretrovir. 2018; 10: 12-17.
- Sibel G, Nural E, Orhan Y, Mehmet K, Mehmet T, et al. The role of goats as reservoir hosts for bovine herpesvirus-1 under field conditions. Trop Anim Health. 2018; 51: 753–758.
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Office International des Epizooties. World Organization for Animal Health, Paris, France. 2018; 1139-1157.
- LeJeune JM, Hart LT, Larson AD, Seger CL. Microimmunodiffusion test for detection of antibodies to infectious bovine rhinotracheitis virus in bovine serum. Am J Vet Res. 1977; 8: 459-463.
- El-Bagoury G, El-Kholy A, Sharawi S, Saad F. Comparing of utilization serological and molecular tools for detection of BoHV-1 in specimens from clinically suspected cattle and buffalo. BVMJ. 2014; 27: 157-165.
- Osman A, Elmenofy W, EL-Kholy A, Soliman M, EL-Gaied L, Abdallah N. Expression of recombinant us9 protein of bovine herpesvirus type1.1 (BoHV- 1.1) in insect cell line and examine its application in an immunoassay for the diagnosis of BoHV-1.1 infected cattle. Egypt J Genet Cytol. 2020; 49: 123-139.
- Seval B, Touraj A, Firat D, Feray A, Aykut O. Molecular and antigenic characterization of bovine herpesvirus type 1 (BoHV-1) strains from cattle with diverse clinical cases in Turkey. Trop Anim Health Prod. 2020; 52: 555– 564.
- Chothe S, Sebastian A, Thomas A, Nissly R, Wolfgang D, Byukusenge M. Whole-genome sequence analysis reveals unique SNP profiles to distinguish vaccine and wild-type strains of bovine herpesvirus-1 (BoHV-1). J Virol. 2018; 522: 27-36.
- Zeedan G, EL-Razik K, Allam A, Abdalhamed A, Zeina H. Evaluations of potential antiviral effects of green zinc oxide andsilver nanoparticles against bovine herpesvirus-1. Adv Anim Vet Sci. 2020; 8: 433-443.
- Dhama K, Chakraborty S, Tiwari R, Verma A, Saminathan M, et al. A concept paper on novel technologies boosting production and safeguarding health of humans and animals. Res Opn Anin Vet Sci. 2014; 4: 353- 370.
- Cork J, Jones R, Sawyer J. Low cost, disposable biosensors allow detection of antibodies with results equivalent to ELISA in 15 min. J Immunol Methods. 2013; 387: 140–146.
- Rana S, Kota S, Samayam P, Rajan S, Srinivasan V. Use of real-time polymerase chain reaction to detect bovine herpesvirus-1 in frozen cattle and buffalo semen in India. Vet Ital. 2011; 47: 313- 322.
- Peili H, Hongmei W, Guimin Z, Chengqiang H, Hongbin H. Rapid detection of infectious bovine Rhinotracheitis virus using recombinase polymerase amplification assays. BMC Vet Res. 2017; 13: 386.
- Thonur L, Maley M, Gilray J, Crook T, Laming E, et al. One-step multiplex real time RT-PCR for the detection of bovine respiratory syncytial virus, bovine herpesvirus 1 and bovine parainfluenza virus 3. BMC Vet Res. 2012; 8: 37.
- Crook T, Benavides J, Russell G, Gilray G, Maley M, Willoughby K. Bovine Herpes Virus 1 abortion: current prevalence in the United Kingdom and evidence of haematogenous spread within the fetus in natural cases. J Vet Diagn. 2012; 24: 662-670.
- Wernine K, Hoffmann B, Kalthoff D, Konig P, Beer M. Development and validation of a triplex real time-PCR assay for the rapid detection and differentiation of wild-type and glycoprotein E-deleted vaccine strains of Bovine herpesvirus type 1. J Virol Methods. 2011; 174: 77–84.
- Zanoli L, Spoto G. Isothermal amplification methods for the detection of nucleic acids in microfluidic devices. Biosens. 2012; 3: 18-43.
- Pawar S, Meshram C, Singh N, Sonwane A, Saini M, et al. Rapid Detection of Bovine Herpesvirus 1 in Bovine Semen by Loop-Mediated Isothermal Amplification (LAMP) Assay. Arch. Virol. 2014; 159: 641–648.
- Sachin S, Chetan D, Niraj K, Arvind A, Mohini S. Rapid detection of bovine herpesvirus-1 in bovine semen by loop-mediated isothermal amplification (LAMP) assay. Arch Virol. 2014; 159: 641–648.
- Rane T, Chen L, Zec H, Wang T. Microfluidic Continuous Flow Digital Loop- Mediated Isothermal Amplification (LAMP). Lab Chip. 2015; 15: 776–782.
- Xu G, Zhao H, Cooper J, Reboud J. A Capillary-Based Multiplexed Isothermal Nucleic Acid-Based Test for Sexually Transmitted Diseases in Patients. Chem Commun. 2016; 52: 12187–12190.
- Alaa A, Khaled A, Hatem S. Rapid detection of BoHV-1 genomic DNA by loop-mediated isothermal amplification assay. J Virol Methods. 2014; 204: 81–85.
- Socha W, Rola J, Urban-Chmiel R, Żmudziński J. Application of loopmediated isothermal amplification (LAMP) assays for the detection of bovine herpesvirus-1. Pol J Vet Sci. 2017; 20: 619–622.
- Nisavic J, Kenezevic A, Stanojevic M, Milic N, Adalj A. Molecular detection of bovine herpesvirus 1 (BoHV-1) in cattle in Serbia. Revue Med Vet. 2018; 169: 180-184.