
Review Article
Austin J Vet Sci & Anim Husb. 2022; 9(4): 1104.
A Review on Antibiotics Residue in Foods of Animal Origin
Tegegne Destaw¹ and Mengesha Ayehu²*
1Amhara Regional State Field Veterinarian Health Practitioner, Ethiopia
2Ethiopian Veterinary Drug and Feed Control and Administrative Authority, Ethiopia
*Corresponding author: Mengesha Ayehu, Ethiopian Veterinary Drug and Feed Control and Administrative Authority, Ethiopia
Received: August 25, 2022; Accepted: September 29, 2022; Published: October 06, 2022
Abstract
Foods of animal origin (example meat, milk, and eggs) samples occasionally, contain excessive amounts of antibiotics drug residues. Usually these are a result of not observing the withdrawal period or from off-label use of an antibiotic. Tetracycline is the most predominantly prescribed antibiotic and of all antibiotic-associated residues, followed by β-lactames. Residues of amino glycosides, Macrolides, and sulfonamides have also been detected. Antibiotics have the potential to cause allergic reactions; penicillin is most commonly implicated, affecting up to 10% of people receiving these drugs therapeutically. Sulfonamides may cause allergic reactions in up to 3% of those using these drugs. Other antibiotics are implicated less often. Concentrations of residual veterinary drugs in foods are not high enough to cause an initial hypersensitive reaction but may cause such an effect in a person who has already become sensitized to the drug. The detection methods of veterinary drug residues in food animal origin constitute a dynamic area in food processing and these include microbiological, immuno-enzymatic and chemical methods. In general, withdrawal period of veterinary drugs must be respected after drugs or medicines have been administered to animals in order to prevent the occurrence of drug residues.
Keywords: Antibiotics; Drug Residues; Food of animal origin
Introduction
Antibiotics are substances either produced naturally by living organisms or produced synthetically in the laboratory, and they are able to kill or inhibit the growth of microorganisms. They can be classified according to their effects as either bactericidal or bacteriostatic and also according to their range of efficacy as narrow or broad in spectrum [35]. Since the discovery and development of the first antibiotics prior to the Second World War, these drugs have played an important role in veterinary and human medicine [17]. Today, antibiotics are used to control, prevent, and treat infection and to enhance animal growth and feed efficiency Currently, approximately 80% of all food-producing animals receive medication for part or most of their lives. The most commonly used antimicrobials in food producing animals are the β-lactames, tetracycline, amino glycosides, lincosamides, Macrolides, and sulfonamides [35].
The use of antibiotics in food-producing animals may leave residues in foodstuffs of animal origin like meat, milk, and eggs [7]. Drug residue is either the parent compound or its metabolites that may accumulate, deposit or otherwise be stored within cells, tissues, organs or edible products (example milk, egg) of an animal following its use to control or treat animal disease [3]. The occurrence of these residues may be due to failure to observe the withdrawal periods, extra-label dosages for animals, contamination of animal feed with the excreta of treated animals, or the use of unlicensed antibiotics, incorrect route of administration and drugs used in species for which they are not intended [28].
Pathogenic microorganisms constitute the most important food related threat to public health and relatively, little is known about food safety in relation to antimicrobial agents. While pasteurization and other forms heat treatment eliminate pathogenic microgranisms from animal source food, these procedures have limited or variable effects on drug residues in animal originated food [33].
Antibiotic residues in foods of animal origin may be the cause of numerous health concerns in humans. These problems include toxic effects, induction of resistant strains of bacteria, immunopathological effects, carcinogenicity (e.g., sulphamethazine, oxytetracycline, and furazolidone), mutagenicity, nephropathy (e.g., gentamicin), hepatotoxicity, reproductive disorders, bone marrow toxicity (e.g., chloramphenicol) and allergy (e.g., penicillin) [38].
Internationally recognized organizations such as the World Health Organization (WHO), Food and Agriculture Organization (FAO), Veterinary Medicine Directorate (VMD) of the European Union (EU) as well as the Food and Drug Administration in the USA (FDA) have set maximum tolerance levels or, Acceptable Daily Intake (ADIs) for humans and withholding times for pharmacologically active substances including antimicrobial agents prior to marketing (Al-Ghamdi et al., 2000). In Africa, in parallel to the incautious use of antibiotics in human medicine, agricultural sectors consume a large portion (50%) of antibiotics in animal farming. However, there is no clear regulation for controlling antibiotic contamination of feedstuffs [34].
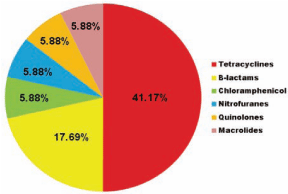
Figure 1: Prevalance of antibiotic residual effect in developing countries.
Antibiotics
Tissue
MRLs
Tetracyclines
(Oxytetracycline, Tetracycline, Chlortetracyclines)Muscle
200 μg/kg
Liver
600 μg/kg
kideny
1200 μg/kg
Milk
100 μg/l
Eggs
400 μg/kg
Source: WHO, 1999
Table 1: The Maximum Residual Limits (MRLs) of tetracycline in animal-derived foods.
In Ethiopia, there is inadequate information on the actual rational drug use pertaining to veterinary drugs. Additionally, there is a clear lack of available information about antibiotic residues in animalderived foods [48].
The objectives of this paper are:
• To review on the scenario in regard to selected antibiotic residues in foods of animal origin.
• To address the public health implications of the effects of these residues, and highlight recommended strategies for controlling the problem.
Antibiotics Residue in Foods of Animal Origin
Foods of animal origin can serve as potential threats to human health if not properly handled. Contamination of these foods with antibiotics residues can result from production at the farm level, transportation and distribution for consumption. These contaminations may arise from diseased animals and unhygienic handling of animal products such as milk and meat. It is estimated that hundreds of millions of people are affected by food-borne diseases of animal origin annually especially in developing countries including Ethiopia [1].
Residues defined as all active ingredients or metabolites of those ingredients that remain in meat or other foodstuffs from the animal to which the medicinal product in question has been administered [21]. Regulation No. 470/2009 of the European Parliament and of the Council defines residues as all pharmacologically active substances, whether active ingredients, excipients or degradation products, and their metabolites, which remain in animal-derived food.
Uses of Antibiotics and Causes of Antibiotic Residues in Food Producing Animals
The introduction of antibiotics to the veterinary field started soon after the use of antibiotics for the treatment of bacterial diseases in humans. The main use of antibiotics in animal rearing was for the treatment and prevention of diseases [19]. Antibiotics are used largely for three purposes in animals, therapeutic use to treat sick animals like mastitis, arthritis, respiratory diseases, gastrointestinal infections, and other infectious bacterial diseases, prophylactic use to prevent infection in animals and as growth promoters to improve feed utilization and production for their growth promoting properties they are routinely used at sub-therapeutic levels as animal feed additives [39].
More recently antibiotics have been used for improved growth, especially in broilers and feed lot animals, antibiotics improve growth rate by thinning of mucous membranes in the gut (which facilitates absorption); alteration of gut motility which enhances assimilation production of favorable conditions for beneficial gut microbes (by destroying harmful bacteria); and partitioning of proteins for muscle growth via cytokine suppression. Antibiotics also favor growth by decreasing the activity of the immune system, reducing the waste of nutrients, and reducing toxin formation. In most cases, however, only young growing animals and poultry are responsive to antibioticmediated health maintenance. This approach actually is problematic as these feed additives are usually used without prescription and for very long periods, in both large and small doses, which leads to drug residues entering animal-derived food [17].
It is a common practice among livestock producers to treat entire groups of livestock, such as birds, fish, or other animals despite there being only a few affected individuals. Such practices unintentionally and unnecessarily expose healthy individuals to antibiotics. Additionally, many livestock producers use Sub therapeutic doses of antibiotics to prevent diseases and this of course will lead to antibiotic residues entering the human food chain. Moreover, antibiotics are prescribed inappropriately in cases of viral infection, which do not respond to such drugs [34].
All licensed antibiotics intended for animal use has clear cessation of use periods, pharmacokinetics and pharmacodynamic profile. Failure to observe the instructions for antibiotic use can lead to antibiotic residues entering animal-derived foods. Improper maintenance of treatment records or a failure to identify treated animals adequately may lead to their omission of these animals. Residues may also transmit vertically to calves consuming milk from cows receiving antimicrobials. Fecal recycling, where the drug excreted in the feces of treated animals contaminates the feed of untreated animals, can be the cause of traces of certain antimicrobial substances being passed on. The most commonly used antimicrobials in food producing animals are the β lactames, tetracyclines, aminoglycosides, lincosamides, macrolides, and sulfonamides [47].
Antibiotics as growth factors: Growth promoters are antibiotics which, when administered in low doses in animal feed, have a preventive effect against certain bacterial infections and modify the composition of the intestinal microbial, improving feed assimilation. The impact of these protective effects on animal production is to accelerate livestock growth [42].
The antibiotic residues occur in food mainly as a result of therapeutic treatment for animals or supplementation of animal feed. A limited amount of these arise by the addition of these antibiotics in preservation of milk, meat, fish and poultry. Microbial resistance to antibiotics results from prolonged ingestion of small amount of contaminated food. prophylactics and growth promoters, In these two cases, the antibiotics are used at a concentration lower than the therapeutics concentrations for a longer period of time, a potentially dangerous practice since it is one of the strongest selective pressures leading to emergence of antibiotic resistance strains of bacteria, induction of allergic reactions in humans and technological problems of fermented meat products (Pavlov And Rusev, 2005).The frequent use of antibiotics may result in drug residues that can be found at different concentration levels in products from animal origin such as milk or meat [27].
Antibiotic Residues in Food of Animal Origin in Ethiopia
In Ethiopia there is low level of control from the government authorities and no quantitative information on antibiotic use in food animals, which are essential for no antibiotic usage in food animals which are essential risk analysis and planning. Besides these missed of antibiotics are highly protected and it is common to see antibiotics being sold in the open market and along the roads by informal venders for administration by farmers themselves and no record keeping at all. All these factors could have greatly contributed for the development of antibiotic resistance and antibiotic residues in food of animal origin. Several research output indicated that there is a significant amount of threat in the use of Tetracyclines in food producing animals. A study conducted in Ethiopia estimated the proportion of Oxytetracycline levels in beef from samples collected from Addis Ababa, Debre Zeit and Adama slaughterhouses. Out of the total 384 samples analyzed for oxytetracycline residues 71.3% had detectable levels. About 48% of the edible tissues had oxytetracycline levels above the recommended maximum limits [35].
Penicillins and Cephalosporines have been widely used in veterinary medicine. Since there is a potential impact of their residues in animal derived food on consumer’s health their monitoring is of paramount importance. A total of 400 bulk milk samples were randomly collected in Debre Zeit dairy farms in 2008 to detect and determine penicillin G residue levels in bulk milk of cows [15].
Major Classes of Antibiotic Drug Residues in Foods of Animal Origin
Antibiotics can be classified according to their effects as either bactericidal or bacteriostatic or depending on the range of bacterial species against which they are active and also according to their range of efficacy as broad or narrow in spectrum. The former fall into four categories: damage to cell membrane function, inhibition of protein synthesis, inhibition of cell wall synthesis, and inhibition of nucleic acid synthesis or function [25].
Tetracyclines: The tetracyclines are the most widely used antimicrobials in veterinary medicine. This is largely due to their affordability, a wide margin of safety and broad-spectrum (Mycoplasma, Gram-positive and Gram-negative bacteria). They are easily administered in mass in either feed or water for poultry (Smith et al., 2000). Among the meat sample collected from the Addis Ababa, Debre Zeit, and Adama slaughterhouses, 93.8%, 37.5%, and 82.1% tested positive for oxytetracycline. The levels of oxytetracycline in muscle from the three slaughterhouses were as follows: Addis Ababa, 108.34 μg/kg; Adama, 64.85 μg/kg; and Debre Zeit, 15.916 μg/ kg. Regarding kidney samples, oxytetracycline levels were found to be 99.02 μg/ kg in Addis Ababa, 109.35 μg/kg in Adama, and 112.53 μg/ kg in Debre Zeit are above the recommended maximum limits [37].
A study conducted on a total of 250 beef samples from five slaughterhouses in and around the city of Nairobi in Kenya showed that114 (45.6%) had detectable tetracycline residues [36]. In Nigeria, All the 25 farms surveyed to determine the prevalence of antibiotics in eggs and retail outlets in Enugu State indicated the presence of significant amount of oxytetracycline residue [23]. Similar researches conducted on milk samples in India [2] and on meat samples in Tabriz and Iran [31] showed the presence of significant amount of tetracycline residues above the acceptable level. High Performance Liquid Chromatography (HPLC) study on total of 500 samples from triceps, gluteal and diaphragm muscles, kidney and liver of beef carcasses from a slaughterhouse in Tabriz showed that 74% of samples had detectable tetracyclines (oxytetracycline, tetracycline, and chlortetracycline) residues. Besides, 5% of kidney and liver samples and 21.7% of all samples contained residues more than the Maximum Residue Limits (MRLs) of the World Health Organization (WHO) [32].
Another study conducted to investigate the presence of Tetracyclines residues in pasteurized, sterilized and raw bovine milk samples in Iran indicated the presence of significant amount of Tetracycline, Oxytetracycline and Chlortetracycline (Tetracyclines) residues, which was higher than the recommended maximum levels (100ng/g) [30]. Similar study conducted on the occurrence of tetracycline residues in cattle milk in five districts in India revealed the presence of tetracycline at a concentration range of 16-134.5 ppb. Among these, three samples exceeded the maximum recommended tetracycline antibiotic residue levels (MRLs) as prescribed by the European Union and the Codex Alimentarius Commission [2].
β-lactames: β-lactames are antibiotics have the beta lactam ring and these include penicillins, cephalosporines, Carbapenems and Monobactams (Aztreonam). Penicillins and Cephalosporines have been widely used in veterinary medicine. Since there is a potential impact of their residues in animal derived food on consumers’ health their monitoring is of paramount importance. A total of 400 bulk milk samples were randomly collected in Debre Zeit dairy farms in 2008 to detect and determine penicillin G residue levels in bulk milk of cows. Out of 400 samples analyzed for antibiotic residue 8.5% were positive for antibiotic residues. The mean residue level of penicillin G was 4.77μg/l. The positive samples which showed residues of penicillin above the WTO (World Trade Organization)/FAO established maximum residue limit of penicillin G with maximum residue limit of 4μg/l, were 20.58% [15].
Similar study that was done in Palestine on 34 raw dairy milk samples where 22.2% were appeared to be above MRLs [26]. Studies conducted in Spain 63% of the analyzed samples were found to be positive for Penicillins and Cephalosporines. Penicillins range between 50-300 μg/kg in beef muscle and 4-30 μg/kg in milk while Cephalosporines range from50 μg/kg to 1000 μg/kg in beef muscle and from 20-100 μg/kg in milk [6].
Aminoglycosides: The aminoglycosides are antibiotics that are widely used in the treatment of bacterial infections such as enteritis and mastitis. They have also been used as feed additives for growth promoter [46]. A study conducted on a total of 240 milk samples, in Turkey showed high levels of aminoglycosides residues (Gentamicin, Streptomycin and Neomycin) above the MRLs which was 200 μg/L, 100 μg/L and 1000 μg/L, respectively [44]. Results from the 2003 FSIS (food safety institution service) National Residue Monitoring Plan also indicate that Neomycin and Gentamicin residues were most commonly detected at violative levels in swine and in a number of cattle meat, particularly calves [17].
Macrolides: Macrolides (example Tilmicosine, Tylosine, Erythromycin, Oleandamycine, Leucomycineycine) are a group of antibiotics that have been widely used to treat many respiratory and enteric bacterial infections in human and animals and their residues can be found in meat, milks, and egg. In Ghana, 35% of the raw milks samples marketed in two major cities, Accra and Kumasi were detected for residues of these drugs and the milk samples contained antibiotic levels above the European Union maximum residue limit [35].
Sulfonamides: Sulfonamides play important role as effective chemotherapeutics of bacterial and protozoan diseases and as growth promoters in veterinary medicine. A study conducted on a total of 497 raw milk samples in Macedonia showed the presence of Sulfonamides which is lower than the acceptable values fixed by World Health Organization. This indicates that toxicological risk associated with the consumption of analyzed milk could not be considered as a public health issue with regards to these drugs [16].
Similar study in Malaysia showed the presence of significant amount of Sulfadiazine, Sulfamethazine, Sulfamethoxazole and Sulfaquinoxaline residues in chicken breast and liver samples [11].
Safety Evaluation for Antibiotics Medication Products Residue
Acceptable daily intake (ADI): It is the amount of a substance that can be ingested daily over a lifetime without appreciable health risk. Calculation of ADI is based on an array of toxicological safety evaluation that takes into acute and long-term exposure to the drug and its potential impact [22].
Maximum residue limit (MRL): It is defined as the maximum concentration of a residue, resulting from the registered use of an agricultural or veterinary chemical, which is recommended to be legally permitted or recognized as acceptable in or on a food, agricultural commodity, or animal feed. The concentration is expressed in milligrams per kilogram of the commodity or milligrams per liter the case of a liquid commodity [8].
Methods for Detecting Antibiotic Drug Residues in Foods of Animal Origin
Screening methodologies: The full procedure and the methodologies for confirmatory analysis are costly in time, equipments and chemicals. In addition, they require trained personnel with high expertise. An ideal screening methodology is easy to use and handle, have low set-up and running costs, high through put., possibility of automatisation, ensure reduced time to obtain the result, have high detection capability (CCb), and good sensitivity and specificity [45].
Immunological Techniques: Antigen and antibody reaction has been used for many years to detect a wide variety of food constituents including substances responsible for adulterations and contaminations. The antigen–antibody interaction is very specific and useful for the detection of residues of chemical and veterinary drugs in animal foods. The most usual technique consists in the enzyme-linked-immunosorbent assay (ELISA) and the detection system is usually based on enzyme-labelled reagents. There are different formats for antigen quantification. In double antibody or sandwich ELISA tests, a primary antibody is bound to the plate well. The antigen of the sample extract added to the well complexes with the bound antibody and remains bound to the plate after washing.
Then, a second antibody labeled with an enzyme such as peroxidase is added to the well followed by a new wash. The quantity of conjugate bound to the plate is detected after incubation with a specific substrate. Color is developed during incubation and measured with a micro-plate reader, which is proportional to the amount of analyte in the sample. In direct competitive ELISA tests, a primary antibody is coated onto the plate wells and incubated with the sample extract containing the antigens. Once the equilibrium is reached, an enzyme-labelled antigen is added. This conjugate will bind to the free binding sites of the primary antibody. Thus, the more antigens in the sample, the lower amount of enzyme-labelled antigen bound. Appropriate specific substrate is added and the plate is incubated for colour development. In this case, there is an inverse relationship between the colour developed and the concentration of the analyte in the sample. Radioimmunoassay (RIA) implies the measure of radioactivity of immunological complex using a counter. Other possibilities include the measure of chemiluminiscence with a luminometer when a chemiluminiscent compound is bound to the antibody or fluorescence with a fluorimeter when a fluorescent compound is used. They allow an enhanced detectability in relation to conventional colorimetry [24].
High Performance Thin-Layer Chromatography (HPTLC): High Performance Thin-Layer Chromatography (HPTLC) has been applied successfully for the qualitative and quantitative detection of multi-residues in food samples even though its use has rapidly decreased during the last decade. Visualization of the components can be performed either by spraying an appropriate chromogenic reagent or under UV light. Quantitative determination is possible through the relative intensity of the spot in the plate, which is measured against that of the internal standard by scanning densitometry. Recent developments allow for automation in a similar way to HPLC with the appropriate equipment. HPTLC has been applied to different residues like thyreostatic drugs, clenbuterol and other agonists, nitroimidazol and sulfonamides in animal tissues. It has also been applied to the analysis of corticosteroids and antibiotics in milk. The spots can consist in the combination of thin-layer chromatography with microbiological detection directly on the plate resulting in enhanced sensitivity. It has been applied to the detection of flumequine in milk. The choice of the detection system is very important for selectivity and sensitivity. Some analytes not detected by absorbance, refractive index or fluorescence may require chemical modifications to render chromophore, fluorescent or UV-absorbing compounds. Usually the detection of multi-residues is based on a solid-phase extraction cleanup followed by filtration and injection into a reversephase HPLC with UV-diode array detection. It has been applied for detection of antibiotics in meat, milk, and eggs. Methyl thiouracils in urine anabolic steroids in nutritional supplements and urine and corticosteroids like dexamethasone in water, feed and meat. A good number of substances with anabolic properties, that can be considered as growth promoters, have been successfully separated and identified for screening purposes in urine [24].
Public Health Significance of Antibiotic Drug Residues in Foods of Animal Origin
In many African countries including Ethiopia, antibiotics may be used indiscriminately for the treatment of bacterial diseases or as feed additives for domestic animals and birds. The ongoing threat of antibiotic contamination is one of the biggest challenges to public health that is faced not only by the African people, but also by the human population worldwide. Such residues are spreading rapidly, irrespective of geographical, economical, or legal differences between countries [50]. The presence of antibiotics in human food is associated with several adverse public health effects including hypersensitivity, tissue damage, gastrointestinal disturbance, and neurological disorders [29,51].
The residues of veterinary drugs or its metabolites in meat and other foods of animal origin may cause adverse toxic effects on consumer’s health [20].
A number of possible adverse health effects of veterinary drug residues have been suggested. The bacteria that usually live in the intestine act as a barrier to prevent incoming pathogenic bacteria from getting established and causing disease. Antibiotics might reduce total numbers of these bacteria or selectively kill some important species (Martínez, 2005). Sensitive individuals may experience allergic reactions to antibiotic residues, particularly penicillin residues, in meat. It is possible that some minor reactions, such as skin rashes, may also have occurred. Estimates of the prevalence of drug sensitivity vary but are estimated to be about 7% in the general population. However, not all of these people experience severe symptoms, and residue levels detected in meat are likely to be below the threshold that would induce a hypersensitive response [17].
The broad-spectrum antibiotics may adversely affect a wide range of intestinal flora and consequently cause gastrointestinal disturbance [12] For example, use of drugs like, flunixin, streptomycin [26] and tylosin in animals, and also use of vancomycin, in humans [12]. The potential hazard of carcinogenic residues of antibiotics are related to their interaction or covalently binding to various intracellular components such as proteins, Deoxyribonucleic Acid (DNA), Ribonucleic Acid (RNA), glycogen, phospholipids, and glutathione [5].
Drug hypersensitivity reaction: Drug hypersensitivity is defined as an immune mediated response to a drug agent in a sensitized patient, and drug allergy is restricted to a reaction mediated by IgE. An allergic or hypersensitive effect following administration of a drug (i.e., drug allergy is quite similar to that typified by allergic response to protein, carbohydrate, and lipid macromolecules. Allergic reactions to drugs may include anaphylaxis, serum sickness, cutaneous reaction, a delayed hypersensitivity response to drugs appear to be more commonly associated with the antibiotics, especially of penicillin (Riedl and Casillas, 2003). About 10% of the human population is hypersensitive to an amount of antibiotics, including penicillin, but in animals, the extent of hypersensitive to, the drug is not well known [9]. Certain macrolides may also in exceptional be responsible for liver injuries, caused by a specific allergic response to macrolide modified hepatic cells [14].
Disruption of Normal Intestinal Flora: The bacteria that usually live in the intestine acts as a barrier to prevent incoming pathogen from being established and causing diseases. Antibiotics may reduce the total number of the bacteria or selectively kill some important species. The broad-spectrum antibiotics may adversely affect a wide range of intestinal flora and consequently cause gastrointestinal disturbance [12]. For example, use of drugs like, flunixin, streptomycin.
Methods of Antibiotics Residue Prevention and Control
There is no doubt that neither humans nor animals can live without antibiotics as they are some of the most effective antimicrobial treatments. However, at the same time the misuse of antibiotics may result in the aforementioned health hazards. Thus, the reduction of antibiotic use constitutes a challenge for the world. In the EU self-monitoring and the control of residues are based on standardized analytical methods. The regulatory framework in force in the EU is based on Directive 96/23/EC, which structures the network of laboratories approved for official residue control, laying down requirements in terms of quality and performance of analytical methods (Decision 2002/657) (EC, 2002).
The residue control method is based on a two-step approach:
A. The detection of residues using sensitive tests with a low rate of false negatives;
B. Followed by confirmation, requiring quantification against the MRL and identification with a low rate of false positives (Mensah et al., 2014).
Hence, the residue prevention strategy is based on preventing entry of violative residues in meat or milk intended for human consumption by proper drug use guide developed for use by both veterinarians and food animal (dairy and beef) producers. All food animals should be maintained in a clean and healthy environment whenever possible. Antibiotic residues are best avoided by implementing management practice and herd health program that keep animals healthy and producing efficiently. Dairy and beef producers should not use or store un- approved drugs, special mixes, or products within adequate labels as unapproved drugs have no data regarding efficacy, safety, or withholding time. The use of prescription drug and a veterinaryclient- patient relationship, which is established hence a veterinarian is closely with the owner in health management of the herd. Before administering or dispensing drugs one has to know the drugs approved for all classes of cattle on the farm and be familiar with approved dosage, route of administration, and withholding times. Institute a workable health record for each animal to record all health related events, including administration of medication. Record the identification of all animals in the permanent health record book. These control points address the conditions under which residue testing should be considered the proper selection and interpretation of tests. The inherent limitation and potential misuse of residue testing and Creating awareness of proper drug use and methods to avoid marketing adulterated products principally educational, total residue avoidance program is based on the objective of improving the livestock producer’s management and quality control of marketing animals with emphasis on avoidance of drug residues [43].
The heat treatment of meat, milk, and eggs may inactivate antibiotic contaminants in feedstuffs and the freezing of animal derived foods may also contribute to the reduction of some antibiotic contamination [50].
In Ethiopia, there are indications on the misuse of antibiotics by health care providers unskilled practitioners and drug consumers. These coupled with rapid spread of resistant bacteria and inadequate surveillance contributed to the problem. Studies on antibacterial resistance and on bacterial infections have shown that emerging antibacterial resistance threatens the management of bacterial infections, however the prevention and containment has received far too little attention. The consequences of these states of affairs include increased mortality, morbidity, costs of treatment, and loss of production in animals [13].
In Ethiopia the control of drugs from the government authorities and information on the actual rational drug use pertaining to veterinary drug use is very limited. In addition, misuses of drugs are common among the various sectors including veterinary and public health. In addition there is lack of awareness and preparedness among the controlling authorities and producers in dealing with the risk of indiscriminate use of antibiotics to the livestock and to the consumers. Food animals slaughtered for domestic and export purposes in the country are not screened for the presence of residues in any of the slaughterhouses in the country. No formal control mechanisms exist to protect the consumers against the consumption of meat and milk products containing harmful drug residues in the country [4].
Conclusion and Recommendation
The use of antibiotics in food of animal origin has caused concern because of their impact on human health. Consumers concern about drug residues continue to erode the demand for animal derived foods and result in disruption of international trade. Clearly at a time when consumer demand for a safe and wholesome food supply has never been greater the need for national regulatory authorities the veterinary profession and the livestock industry to assert a strong cooperation for food safety have never been more critical. Formal training in the area of residue prevention has been limited at a time when advances are rapidly reshaping the way that food safety programs operate. The development of rapid immunodiagnostic tests for drug residues has allowed the monitoring of much greater numbers of animal products prior to reaching the food supply. So together with these food safety programs surveillance systems should be in place to ensure that standards of animal products are being met.
Based on the above conclusion the following recommendations are forwarded.
• The effective prevention of infectious diseases and the adoption of strict hygiene standards and rearing skills should be reducing antibiotics residues, particularly in the veterinary field.
• Strict national legislation must be passed around the world to avoid the unnecessary use of antibiotics.
• Avoid using antibiotics in the veterinary field without a veterinarian’s prescription.
• Avoidance of antibiotics that have not clearly documented pharmacokinetic and pharmacodynamic properties.
• Antibiotics use in food animals should be reduced by improving animal health through bio security measures.
• Antibiotics should be administered to food animal’s only professionals.
• Narrow-spectrum antibiotics should be the first choice when antibiotic therapy is justified.
• Use of antibiotics as growth promoters should be prohibited.
• Antibiotics should be used only therapeutically and they should be given to sick animals based on the results of resistance surveillance.
• Veterinary extensions and practices must be promoted in Ethiopia.
• Study should be conducted in Ethiopia to determine the level of antibiotic resistant pathogens in different food items.
References
- Abdou EA. Application of food safety in developing countries. Epidemiology of meat borne and milk borne infections in the Mediterranean region. Inf Circ WHO/Mediter Zoon. Control Centre. 2002; 54: 6-9.
- Abhishek G, Gill JPS, Aulakh RS, Bedi JS. ELISA based monitoring and analysis of tetracycline Residues in cattle milk in various districts of Punjab. Punjab, India. School of Public Health and zoonoses. Vet World - 141004. 2014; 7: 26-9.
- Adams H. Iowa Univ.press. 7th ed.1995: Veterinary pharmacology and therapeutics; 19: 416-22.
- Addisalem H, Bayleyegn M. Tetracycline residue levels in slaughtered beef cattle from three slaughterhouses in Central Ethiopia. GlobVet. 2012; 8: 546- 54.
- Aiello SE, Lines PR, Kehn CM. Anthelmintics in the merck veterinary manual. 9th ed; 2005. Co.Ioc.NJ. 2111-24.
- Alexandra J, Rameshwari A, Rafael P, Gultekin G, Edyta G, Dolores B, et al. Residues of β lactams and quinolones in tissues and milk samples. Confirmatory analysis by liquid chromatography–mass spectrometry. Spain, Barcelona. 2010; 2: 109-22.
- Bevil RF. Factors influencing the occurrence of drug residues in animal tissues after the use of antimicrobial agents in animal feeds. JAVMA. 1989; 185: 1124-6.
- Boisseau J. Basis for the evaluation of the microbiological risks due to veterinary drug residues in food. Vet Microbiol. 1993; 35: 187-92.
- Boothe DM, Reevers PT. Pharmacology introduction. In: Aiello SE, Moses MA, Steigerwald MA, editors The merck veterinary manual. Merck Publishing Group. Pp: 10; 2012.
- Carlet J, Jarlier V, Harbarth S, Voss A, Goossens H, Pittet D, et al. Ready for a world without antibiotics? The Pensières antibiotic Resistance Call to Action. Antimicrob Resist Infect Control. 2012; 1: 11.
- Cheong C, Hajeb P, Jinap S, Ismail-Fitry M. Sulfonamides determination in chicken meat products from Malaysia. Int Food Res J, 43400 UPM. 2010; 17: 885-92.
- Cotter PD, Stanton C, Ross RP, Hill C. The impact of antibiotics on the gut microbiota as revealed by high throughput DNA sequencing. Discov Med. 2012; 13: 193-9.
- DACAE. and MSH/SPS. Antimcirobials use, resistance and containment baseline survey syntheses of findings. A report published by Drug Administration and Control Authority of Ethiopia. Addis Ababa, Ethiopia; 2009.
- Darwish WS, Eldaly EA, El-Abbasy MT, Ikenaka Y, Nakayama S, Ishizuka M. Antibiotic residues in food: the African scenario. Jpn J Vet Res. 2013; 61: S13-22.
- Desalegne AS. Detection and determination of oxytetracycline and penicillin Gantibiotic residue levels in bovine bulk milk from Debrezeit and Nazareth dairy farms. Ethiopia: Faculty of Veterinary Medicine, Addis Ababa University: Human Resource Management Academic Research Society; 2008: 32.
- Dimitrieska S, Elizabeta H-M, Zehra SDB, Sekulovski P, Uzunov R. Screening of veterinary drug residues in milk Individual Farms In Macedonia. Original Scientific Article Mac. Vet Res. 2010; 34: 5-13.
- Doyle ME. Food research institute. WI: University of Wisconsin–Madison Madison; 2006: 53706.
- Doyle ME. Veterinary drug residues in processed meats-potential health risk. FRI Briefings. Madison: Food Research Institute, University of Wisconsin- Madison. 2006; 556: 415-22.
- Draisci R, delli Quadri F, Achene L, Volpe G, Palleschi L, Palleschi G. A new electrochemical enzyme linked immunosorbent assay for the screening of macrolide antibiotic residues in bovine meat. Analyst. 2001; 126: 1942-6.
- EFSA. Opinion of the scientific panel on contaminants in the food chain on a request from the European Commission related to hormone residues in bovine meat and meat products. EFSA J. 2007; 510: 1-62.
- European Commission. Council Directive 81/852/EEC of 28 September 1981 on the approximation of the laws of the Member States relating to analytical, pharmaco-toxicological and clinical standards and protocols in respect of the testing of veterinary medicinal products. Off J Eur Commun. 1981; 317: 16- 28.
- European Community (EC). Notice to applicant and note for guidance. Establishment of maximum residue limits for residue of veterinary medicinal products in food stuffs of animal origin. 2001; 4-10.
- Ezenduka EV, Oboegbulem SI, Nwanta JA, Onunkwo JI. Prevalence of antimicrobial residues in raw table eggs from farms and retail outlets in Enugu State, Nigeria. Trop Anim Health Prod. 2011; 43: 557-9.
- Toldra F, Reig M. Methods for rapid detection of chemical and veterinary drug residues in animal foods. Trends Food Sci Technol. 2006; 17: 482-9.
- Gustafson RH. Historical perspectives on regulatory issues of antimicrobial resistance. Vet Hum Toxicol. 1993; 35: 2-5.
- Ibrahim MAlZ. Detection of β-lactams and tetracyclines antimicrobial residues in raw dairy milk for human consumption in Palestine, Nablu. Jackson, BA. (1980). Faculty of Veterinary Medicine, a Najah National University.Food and Drug Administration, Center for Veterinary Medicine. Hosted at University of Nebraska Lincoln. Jam Vet Med Assoc. 2012; 176-1141.
- Kempe M, Verachtert B. Cartridges with molecularly imprinted recognition elements for antibiotic residues monitoring in milk fream Pure and Applied Biochemistry. Lunds Universitet centre for chemistry and chemical engineering getingevagen. Lund, Sweden. 2000: 1-10.
- Kurwijila LR, Omore A, Staal S, Mdoe NSY. Investigation of the risk of exposure to antimicrobial residues present in marketed milk in Tanzania. J Food Prot. 2006; 69: 2487-92.
- Lee MH, Lee HJ, Ryo PD. Public health risk chemicals and antibiotic residuesreview. Asian-Aust. Ani J Sci. 2000; 14: 402-13.
- Mehran M, Abbasi H, Babaei MA, Ashraf N, Mahboob N. Simultaneous Determination of Tetracyclines Residues in Bovine Milk Samples by Solid Phase Extraction and HPLC-FL Method. Adv Pharm Bull. 2011; 1: 34-39.
- Mehran M, Abbasi H, Babaei MA, Ashraf N, Mahboob N. Simultaneous Determination of Tetracyclines Residues in Bovine Milk Samples by Solid Phase Extraction and HPLC-FL Method. Adv Pharm Bull. 2009; 1: 3.
- Mehran M, Abbasi H, Babaei MA, Ashraf N, Mahboob N. Simultaneous determination of tetracyclines residues in bovine milk samples by solid phase extraction and hplc-fl method. Advanced pharmaceutical bulletin. 2007; 1: 3.
- Moat WA. Inactivation of antibiotics by heating in foods and other substrate-a review. J Food. 1988; 51: 491-7.
- Mohamed Ms. antibiotic residues in commercial layer hens in Khartoum state, Sudan; faculty of veterinary science, university of pretoria. South Africa; 2010.
- Morshdy AE, El-Atabany AI, Hussein MA. Wageh Sobhy Darwish WS. oxytetracycline residues in bovine carcasses slaughtered at Mansoura Abattoir, Egypt. Jpn J Vet Res. Inpress. 2013.
- Muriuki FK, Ogara WO, Njeruh FM, Mitema ES. Tetracycline residue levels in cattle meat from Nairobi slaughter house in Kenya. J Vet Sci. 2001; 2: 97-101.
- Myllyniemi AL, Rannikko R, Lindfors E, Niemi A, Bäckman C. Microbiological and chemical detection of incurred penicillin G, oxytetracycline, enrofloxacin and ciprofloxacin residues in bovine and porcine tissues. Food Addit Contam. 2000; 17: 991-1000.
- Nisha AR. Antibiotics residues a global health hazard. Vet World. 2008; 2: 375-7.
- Okerman L, Van Hoof J. WD. Evaluation of the European four-plate test as a tool for screening antibiotic residues in meat samples from retail outlets. J. Assoc. Off. Anal. Cnem. 1998; 81: 51-6.
- Pavlov ALL, Rusev V. Studies on the residue levels of tobramycin in stored poultry products. Trakfa J Sci. 2005; 3: 20-2.
- Reyes-Herrera I, Schneider MJ, Cole K, Farnell MB, Blore PJ, Donoghue DJ. Concentrations of antibiotic residues vary between different edible muscle tissues in Poultry. J Food Prot. 2005; 68: 2217-9.
- Sanders P. Antibiorésistance vétérinaire medicine: enjeux de santé publique et de santé animale. Bull. Acad vét Fr. 2005; 158: 137-42.
- Scippo ML, Degand G, Duyckaerts A, Maghuin-Rogister G, Delahaut P. Control of the illegal administration of natural steroid hormones in the plasma of bulls and heifers. Analyst. 1994; 119: 2639-44.
- Seyda E, Ayhan F. Determination of antibioticresidues in milksamples. Department of pharmacology and toxicology, faculty of veterinary medicine, Ankara – TURKEY Ankara University. TR(ArticleCode): KVFD-2009-1. 2010; 06110.
- Shankar S, Prabhu M, Chandan C, et al. Rapid Methods for detection of Veterinary Drug residues in Meat. Vet World. 2010; 3: 241-246.
- Stead DA. Current methodologies for the analysis of aminoglycosides. J Chromatogr B Biomed Sci Appl. 2000; 747: 69-93.
- Sundlof SF. Drug and chemical residues in livestock. Vet Clin North Am Food Anim Pract. 1989; 5: 411-49.
- Tajick M, Rahimian H, Alizadeh A, Rezaeian J. Analysis of isolated lipids from sclerotia Assoc off. Agric Chem. 2002; 34: 652-61.
- Vanpeteguem C, Daeselaire E. Residues of growth promoters. L Med. 2nd ed, hand book of food analysis. 2004; 137-63.
- Wageh SD, Elsaid AE, Mohamed TE, Yoshinori I, Shouta N, Mayumi I.Antibiotic residues in food: the African scenario: a review Japanese Journal of Veterinary Research. 2013; 61: 13-22.
- Wassenaar TM. The use of antimicrobial agents in veterinary medicine and implications for human health. Crit Rev Microbiol. 2005; 31: 155-69.