
Special Article - Pig Health
Austin J Vet Sci & Anim Husb. 2022; 9(5): 1109.
Application of a β -Mannanase Enzyme in Diets with a Reduced Net Energy Content Results in Reduced Production Costs Per Kg of Carcass Weight
Vangroenweghe Frédéric1,2*
¹Elanco, BU Food Animals, Plantijn en Moretuslei 1-3rd Floor, 2018 Antwerpen, Belgium
²Ghent University, Faculty of Veterinary Medicine, Unit of Porcine Health Management, Merelbeke, Belgium
*Corresponding author: Frédéric Vangroenweghe, BU Food Animals, Elanco Benelux, Plantijn en Moretuslei 1, 2018 Antwerpen, Belgium
Received: October 03, 2022; Accepted: October 28, 2022; Published: November 04, 2022
Abstract
β-Mannans are strongly anti-nutritive polysaccharide fibres found in most vegetable feed ingredients. They belong to the hemicellulose fraction and have a backbone composed entirely of mannose, as in mannans and galactomannans, or of mannose and glucose, as in glucomannans and galacto-glucomannans. The estimated content of soluble β-mannans in common fattening diets is only 0.20-0.35%, and in vitro studies have demonstrated that as little as 0.05% soluble β-mannan in feed can elicit a strong innate immune response. This innate response is often referred to as a feed induced immune response or FIIR, which suppresses growth to protect the liver and reserve energy and nutrients for high priority immune functions. Hemicell HT (Elanco Animal Health) is a β-mannanase enzyme for animal feed that breaks down β-mannans and thereby prevents economic losses from the wasteful immune response to β-mannans. The aim of the present study was to compare pig performance and carcass parameters on a control diet and a reformulated diet with lower energy content - a 65 kcal reduction in net energy per kg – and inclusion of a β-mannanase enzyme. An eighteen-week feeding trial was conducted on a commercial fattening unit with DanBred x Belgian Piétrain pigs starting at 10 weeks of age. Standard two-phase control diets (Control group) were compared to reformulated diets with an energy reduction of 65 kcal NE/kg and inclusion of a β-mannanase enzyme (Hemicell HT; Elanco) at 300 g/tonne (Enzyme treated group). Standard production and health data were collected including days in fattening, bodyweight at start, at 28 days and at 127 days (prior to slaughter), feed intake, mortality and antibiotic use. Additionally, Average Daily Weight Gain (ADWG), Average Daily Feed Intake (ADFI) and Feed Conversion Rate (FCR) were calculated from the collected data. Furthermore, pigs were slaughtered on separate slaughter days to reliably collect relevant carcass parameters at slaughterhouse level. The data were analysed using JMP 15.0 statistical program. Overall, performance data did not differ significantly between trial groups in both Phase 1 and Phase 2, except for mortality that was significantly higher during Phase 1 in the Control group (3.19% vs. 0.00% in the Enzyme treated group). Carcass quality did only significantly (P < 0.05) differ for muscle depth (73.58 ± 0.66 mm vs. 75.33 ± 0.59 mm in the Control and Enzyme treated group, respectively). Following calculation of feed costs per kg carcass weight and considering the cost of enzyme inclusion, Enzyme treated pigs had € 0.033 lower feed costs per kg carcass weight as compared to Control pigs. The trial demonstrated that inclusion of Hemicell HT in reformulated diets with a lower energy content (65 kcal NE/kg) was able to degrade β-mannans in diets and therefore maintain production performance and overall carcass quality. Reduction in mortality may indicate an improved overall immunity due to decreased FIIR by the breakdown of β-mannans in the feed. Inclusion of a β-mannanase enzyme resulted in an overall reduction in production costs of € 0.033 per kg of carcass weight, which is an additional advantage considering the current feed prices globally.
Keywords: β-mannanase; Grow-finishing pigs; Net energy reduction; Performance; Carcass quality
Introduction
Feed cost is by far the most expensive production factor in the swine industry and continues to increase due to price volatility of different crucial ingredients on the global market. Besides the concerns on protein supply and quality, energy supply and cost for swine diets remains a major issue under the current market situation. Recent calculations [September 2022] reveal an additional 100 kcal net energy have an estimated economical value of € 19.50 per tonne of formulated diet. Polysaccharides, polymers of monosaccharides linked by glycosidic bonds, are major components of feed ingredients frequently used as an energy source in the swine industry. Starch, a polymer of glucose units linked by α-(1-4) with a few α-(1-6) bonds, is digested in the small intestine of pigs through endogenous enzyme activity. Non-Starch Polysaccharides (NSPs) are fibrous materials found in plant cell wall which include celluloses, hemicelluloses, pectins and oligosaccharides. Monogastric animals such as pigs do not possess endogenous enzymes capable of cleaving and digesting β (α)-linked NSPs, such as β-mannans. β-mannans in swine diets might hinder utilization of nutrients [1].
β-Mannan is an antinutritive factor found in common feed ingredients [2], which has received increasing attention in recent years. β-Mannans are linear polysaccharides composed of repeating units of β-1,4-mannose and α-1,6-galactose and/or glucose units attached to the β-mannan backbone [3,4]. They are unsuitable in pig feed in high concentrations due to their antinutritive properties resulting in the stimulation of the innate immune response. In fact, the innate immune cells identify pathogens using distinct molecules, called Pathogen Associated Molecular Patterns (PAMP), expressed on the pathogen surface [5]. Binding of PAMPs to Pathogen Recognition Receptors (PRR) present on innate immune cells, results in the release of innate defense molecules such as reactive oxygen and nitrogen species, bacteriolytic enzymes, antimicrobial peptides and complement proteins [6]. These PAMPs include complex polysaccharides such as β-mannan [5]. Therefore, β-mannans from feed can create a false signal about the presence of pathogens in the gut, eliciting an unwarranted immune activation [7,8], which is also known as a feed-induced immune response FIIR; [9]. This recognition mistake leads to a futile immune response that causes energy and nutrients to be wasted [4]. Hydrolysis of these β-mannans through inclusion of exogenous β-mannanase enzymes can reduce and potentially eliminate their ability to induce FIIR.
In poultry, the inclusion of dietary β-mannanase has been shown to improve daily gain and feed efficiency, while decreasing digesta viscosity [10], and to upregulate a broad range of metabolic functions related to digestion, metabolism and immunity [9]. Moreover, beneficial effects of β-mannanase addition in chickens, challenged with Eimeria sp. and Clostridium perfringens, were observed with improved performance and reduced lesion scores in diseasechallenged animals [11].
Supplementation of β-mannanase to maize-soybean meal (SBM)-based diets improved growth performance, whereas nutrient digestibility remained at a similar level [12]. Moreover, supplementation of β-mannanase to low- and high-mannan diets has the potential to improve the performance of growing pigs. The improved overall pig performance following supplementation of β-mannanase to corn-SBM-PKM diets might be due to increased adjusted ileal digestibility of different amino acids [13-15]. Others concluded that β-mannanase improved growth performance in both weanling and growing-finishing pigs on corn-SBM diets [16,12,17] with minimal effects on nutrient digestibility [12].
The objective of the current study was to evaluate the effects of β-mannanase supplementation to grow-finishing diets with reduced net energy content of 65 kcal/kg on performance and slaughter data of grow-finishing pigs.
Materials and Methods
Description of Experimental Farm
The field trial was performed on a conventional fattening unit in Belgium with 2 compartments of 8 pens each, in both grow and finishing facility. Ventilation of compartments was performed mechanically with a central fan and an air inlet through the door. All pens had partially slatted concrete floors. Water was distributed through a nipple in the feeder.
Each pen was equipped with a dry feeder filled with meal. Feed intake was calculated at group level (1 feed bin per treatment group).
Experimental design
Treatment groups: Two treatment groups were enrolled during the trial. The Control group received a standard 3-phase feeding schedule, whereas the Enzyme treated group received a reformulated 3-phase diet with a reduction of 65 kcal NE per kg feed and supplementation of a β-mannanase enzyme. Both groups were blinded to the farm personnel and only distinguished by letter codes (A,B). The 2 groups were equally distributed over the 2 compartments and 16 pens, containing each 11-12 pigs. Between Phase 1 and Phase 2, pigs were moved to another building to comply with a stocking density of 0.45 m² per pig during the grow phase and 0.80 m² per pig during the finishing phase at a stocking density of 0.8 m².
Experimental diets
Pigs were fed a pelleted 3-phase diet consisting of starter (0-28 d), grower (29-63 d) and finisher (64-127 d) diet in both treatment groups. The Enzyme treated group differed predominantly from the Control group by reduction in net energy content of 65 kcal per kg feed in starter, grower, and finisher diets. This reduction in net energy was realized through reduction of soya oil percentage in the Enzyme treated group by 0.8%. Addition of a β-mannanase enzyme (Hemicell HT; Elanco, Indianapolis; IN) was performed at an inclusion level of 300 g per tonne of feed, according the manufacturer’s instructions. All other enzymes (xylanase and phytase) in the diets remained at the same levels in both treatment groups.
Experimental animals
DanBred x Belgian Piétrain piglets were obtained from a conventional commercial sow farm in Belgium operating in a 4-week batch-management system, which is known to improve overall pig health [18]. Pigs were vaccinated to protect against Mycoplasma hyopneumoniae and Porcine Circovirus type 2 (PCV-2) during the suckling period. All pigs originated from the same farrowing batch and were approximately 10 weeks of age at the start of the feed trial.
Performance data collection
Pig Body Weight (BW) at pen level was measured at 0, 28 (end of Phase 1) and 127 days (end of Phase 2) after arrival on the finisher farm. Feed provision (ad libitum) was recorded per period for the entire treatment group. For both phases (Phase 1 = start; Phase 2 = grow-finishing), average daily weight gain (ADFI; expressed as g/d), average daily feed intake (ADWG; expressed as g/d) and feed conversion rate (FCR; expressed as kg feed per kg of weight gain) were calculated.
Veterinary treatments
Individual antibiotic treatments could be performed as needed due to the critical state of the piglet and in case of a broader health issue in the barn, group treatment could performed. Both types of treatment were always performed according to the clinical criteria of the farm veterinarian. The same veterinary products, active ingredients, formulations, and dosages (ml/kg) were used throughout the entire study period. Individual antibiotic treatments or group treatments were recorded daily by date, product, dose, ID number of treated piglets, presumed cause of treatment, and number of times the treatment was repeated. For all treatment groups, the same antibiotic product and dosage was applied for the same indication.
Data management and statistical analysis
Data were hand-recorded by the farm personnel and stored in MS Excel on OneDrive at the end of each day. Following the end of the finisher phase, data were extracted from Excel into JMP 15.0 and the blinded letter-coded treatments were unblinded to reveal the respective treatment groups. Calculations, exploratory data analysis and quality review, and subsequent statistical analysis were all performed in JMP 15.0. All data are presented as means with their standard error of the mean (SEM, where available). All means were tested for significant differences (P < 0.05) using a post-hoc T-test.
Results
Pig weight and average daily weight gain
Pigs were transferred to the grow-finishing unit at 10 weeks of age and an average weight of 22.16 ± 1.61 and 22.32 ± 1.58 kg for the Control and Enzyme treated group, respectively. At the end of Phase 1 (0-28 days), Control pigs were slightly, but not-significantly (P > 0.05) heavier compared to Enzyme treated piglets (40.73 ± 2.86 kg vs. 40.01 ± 1.01 kg, respectively). After the subsequent transfer to the finishing pens, some additional pigs, that were fed the same diets in Phase 1 and had a similar weight (n = 4 and 2 in Control and Enzyme treated group, respectively), were introduced to compensate for mortality and to optimize the pen occupation. Therefore, at the start of Phase 2 (28-127 days), Control pigs were still slightly, but notsignificantly (P > 0.05) heavier as compared to the Enzyme treated pigs (41.22 ± 1.51 kg vs. 40.07 ± 0.88 kg, respectively). At loading, the Control pigs remained slightly, but not-significantly (P > 0.05) heavier as compared to the Enzyme treated pigs (134.55 ± 1.15 kg vs. 133.91 ± 2.16 kg, respectively) (Table 1).
Control
Hemicell HT
Phase 1
Number of pigs enrolled
94
94
Mortality (# / %)
3 (3.19%)*
0 (0%)*
Weight d0 (kg)
22.16 ± 1.61
22.32 ± 1.58
Weight d28 (kg)
40.73 ± 2.86
40.01 ± 1.01
Phase 2
Number of pigs enrolled
95
96
Mortality (# / %)
1 (1.05%)
2 (2.10%)
Weight d0 (kg)
41.22 ± 1.51
40.07 ± 0.88
Weight d99 (kg)
134.55 ± 1.15
133.91 ± 2.16
Table 1: Performance parameters (mean ± SEM) in Phase 1 (0-28 days) and Phase 2 (28-127 days) of the fattening period for pigs fed a control diet (Control) or a reformulated diet with an energy reduction of 65 kcal NE/kg and inclusion of a beta-mannanase Hemicell HT (Elanco) at 300 g/T (Enzyme). * Indicates significant differences (P < 0.05) between both study groups.
Control
Enzyme
Number of pigs slaughtered
91
90
Hot carcass weight (kg)
106.55 ± 1.18
107.26 ± 1.05
Lean meat (%)
62.49 ± 0.32
62.89 ± 0.37
Backfat thickness (mm)
9.80 ± 0.34
9.60 ± 0.37
Muscle depth (mm)
73.58 ± 0.66*
75.33 ± 0.59*
Production cost per kg carcass weight
- 0.033c€
Table 2: Slaughter data (mean ± SEM) and difference in production cost per kg meat harvested for pigs fed a control diet (Control) or a reformulated diet with an energy reduction of 65 kcal NE/kg and inclusion of a beta-mannanase Hemicell HT (Elanco) at 300 g/T (Enzyme). *Indicates significant differences (P < 0.05) between both study groups.
Average daily weight gain in Phase 1 was 17 g/d lower in Enzyme treated piglets as compared to Control piglets. In Phase 2, no difference in ADWG could be observed between Enzyme treated and Control piglets. Average daily weight gain was not significantly (P > 0.05) different between both group in neither of the two feeding phases (Figure 1).
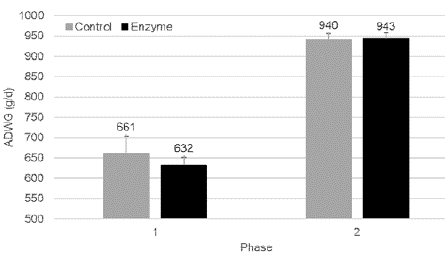
Figure 1: Average daily weight gain (g/day; mean ± SEM) in Phase 1 (0-28
days) and Phase 2 (28-127 days) for pigs fed a control diet (Control) or a
reformulated diet with an energy reduction of 65 kcal NE/kg and inclusion of
a beta-mannanase Hemicell HT (Elanco) at 300 g/T (Enzyme). No significant
differences (P > 0.05) were observed between both study groups.
Feed intake and feed conversion rate
Feed intake in Control pigs was slightly higher (+ 35 g/d) in Phase 1 as compared to Enzyme treated piglets. In Phase 2, both treatment groups consumed exactly the same amount of feed (Figure 2).
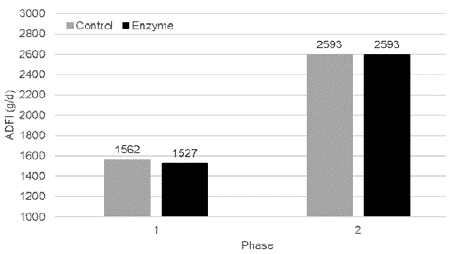
Figure 2: Average daily feed intake (g/day; mean) in Phase 1 (0-28
days) and Phase 2 (28-127 days) for pigs fed a control diet (Control) or a
reformulated diet with an energy reduction of 65 kcal NE/kg and inclusion of
a beta-mannanase Hemicell HT (Elanco) at 300 g/T (Enzyme). No significant
differences (P > 0.05) were observed between both study groups.
Feed conversion rate in Phase 1 was not different between both treatment groups (2.41 vs. 2.42 in Control and Enzyme treated group, respectively). In phase 2, FCR was equal (2.75) in both treatment groups (Figure 3).
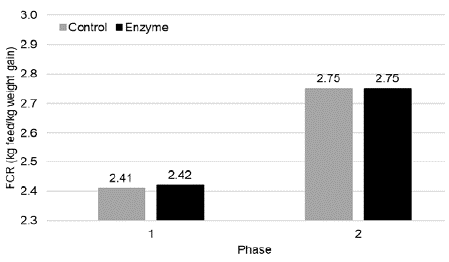
Figure 3: Feed conversion rate (kg feed/kg weight gain; mean) in Phase 1
(0-28 days) and Phase 2 (28-127 days) for pigs fed a control diet (Control) or
a reformulated diet with an energy reduction of 65 kcal NE/kg and inclusion of
a beta-mannanase Hemicell HT (Elanco) at 300 g/T (Enzyme). No significant
differences (P > 0.05) were observed between both study groups.
Mortality
Data related to mortality are given in Table 1. In summary, the Control group had a significantly higher (P < 0.05) mortality of 3.19% (n = 3) as compared to 0.00% (n = 0) in the Enzyme treated group during Phase 1. During Phase 2, no significant difference in mortality could be observed between the Control (n = 1; 1.05%) and the Enzyme treated (n = 2; 2.10%) group, respectively.
Antimicrobial treatment
No antimicrobial treatments were administered throughout the entire study period.
Slaughter data and production cost per kg of carcass weight produced
In total, 91 pigs in the Control group and 90 pigs in the Enzyme treated group were slaughtered. Hot carcass weight, lean meat percentage and backfat thickness were not significantly (P > 0.05) different between both treatment groups. Enzyme treated pigs had a significantly (P < 0.05) higher muscle depth (75.33 ± 0.59 mm) as compared to the Control pigs (73.59 ± 0.66 mm).
The production cost per kg of carcass weight was € 0.033 lower in the Enzyme treated as compared to the Control group. This implies a total difference in production cost of € 3.54 per pig at 107.26 kg carcass weight.
Discussion
In the current study, we reduced the net energy content by 65 kcal per kg feed in a 3-phase diet through reduction of soya oil inclusion, whereas the remaining components were kept constant as compared to the Control diet. All diets had a sufficiently high β-mannan content, a known anti-nutritive factor [2], which may stimulate an innate immune response through their resemblance with PAMPs [5]. This activation has been called FIIR [9] and leads to an unnecessary immune activation, causing energy and nutrients to be wasted [4]. Therefore, 300 g/tonne of an exogenous β-mannanase enzyme (Hemicell HT; Elanco, Greenfield, IA) was added to hydrolyze these antinutritive β-mannans in the trial feed. The results in Phase 1 and Phase 2 demonstrated no significant differences in the measured (pig weight, feed intake) or calculated (ADWG, ADFI, and FCR) performance parameters between both treatments. Although minor numerical differences were observed, the overall result confirmed that the addition of an exogenous β-mannanase to adapted formulations with a reduction in net energy of 65 kcal per kg feed allowed them to perform equal to the standard Control diets.
Several studies concluded that β-mannanase improved growth performance in both weanling and growing-finishing pigs on corn- SBM diets [12,16,17]. A diet with a 150 kcal/kg reduction in digestible energy supplemented with β-mannanase outperformed in weight gain and feed efficiency [16]. Others have also observed the energy sparing effect from supplementation of β-mannanase. Supplementation to a common nursery diet resulted in similar effects on performance of a comparable diet supplemented with 2% soya oil [12].
The difference in mortality between both treatment groups might be explained by the activation of FIIR through the presence of sufficiently high levels of β-mannans in the Control group, whereas these β-mannans were degraded by the inclusion of a heat-tolerant β-mannanase in the Enzyme treated group. A reduction in FIIR might help the immune system to focus on conventional immune challenges at the intestinal or respiratory level and therefore mount a more efficient immune reaction towards these challenges, resulting in a lower chance of tissue damage and subsequent mortality. This is in accordance with a previous study in broilers, where inclusion of β-mannanase also resulted in a lower mortality [19].
Slaughter data revealed only a significant difference in muscle depth between both treatment groups, whereas all other carcass quality parameters were similar. The better muscle depth in the Enzyme treated group might be explained by the more optimal protein deposition at the muscle level, mainly due to the lower FIIR, which predominantly leads nutrients, including protein, towards an unwanted, unnecessary intestinal immune response in reaction to the present β-mannans in the diet.
In conclusions, the current study results suggest that the use of an exogenous heat-tolerant β-mannanase allowed a reduction in net energy of 65 kcal per kg feed to be applied in all phases of a 3-phase grow-finishing diet without adverse effects on intestinal health, overall performance or carcass quality.
Acknowledgements
The author greatly acknowledges the swine farmer, farm veterinarian and feed company nutritionist for their participation in the study.
Declarations
Ethics approval and consent to participate - Field trial with an EFSA approved feed supplement for use in swine. No additional ethical approval needed. Consent to participate was obtained following full information of farmer on the protocol to be carried out.
Consent for publication - Not applicable.
Availability of data and material - The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests - The authors declare that they have no competing interests.
Funding - The study was funded by Elanco Animal Health, which facilitated the conduct of the field trial.
Author’s contributions - FV was involved in study design, data collection, data analysis and manuscript preparation.
Author’s information - FV is currently a Principal Technical Advisor Swine and Nutritional Health for Benelux / UK&ROI within Elanco Animal Health. He holds a DVM, a Master in Veterinary Public Health and Food Safety, a PhD in Veterinary Sciences, a PhD in Applied Biological Sciences and an EBVSTM European Specialist in Porcine Health Management. He is currently also Resident in the American Board of Veterinary Practitioners -Swine Health Management. He has a specific interest in swine intestinal health and specific approaches to improve intestinal health through nonantibiotic solutions.
References
- Rainbird AL, Low AG, Zebrowska T. Effect of guar gum on glucose and water absorption from isolated loops of jejunum in conscious growing pigs. Br J Nutr. 1984; 52: 489-498.
- Ferrel J, Anderson DM, Hsiao HY. Content of Soluble Non-Starch Polysaccharides β-Mannan and Xylan in Legume Meals, Non-Legume Meals, and Cereal Grains or Cereal Grain by-products. Journal Animal Science. 2014; 92.
- Jackson ME, Geronian K, Knox A, McNab J, McCartney E. A dose-response study with the feed enzyme β-mannanase in broilers provided with cornsoybean meal based diets in the absence of antibiotic growth promoters. Poult Sci. 2004; 83: 1992-1996.
- Hsiao H-Y, Anderson DM, Dale NM. Levels of β-mannan in soybean meal. Poult Sci. 2006; 85: 1430-1432.
- Forsberg NE, Wang Y. Nutrition and immunity in dairy cattle: implications to hemorrhagic bowel syndrome. Proc Mid-South Rum Nutr Conf. 2006; 11-20.
- Sukhithasri V, Nisha N, Biswas L, Kumar VA, Biswas R. Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions. Microb Res. 2013; 168: 396-406.
- Anderson DM, Hsiao HY, Dale NM. Identification of an inflammatory compound for chicks in soybean meal. Poult Sci. 2008; 88: 153-157.
- Li Y, Chen X, Chen Y, Li Z, Cao Y. Effects of β-mannanase expressed by Pichia pastoris in corn soybean meal diets on broiler performance, nutrient digestibility, energy utilization and immunoglobulin levels. Anim Feed Sci Technol. 2010; 159: 59-67.
- Arsenault RJ, Lee JT, Latham R, Carter B, Kogut MH. Changes in immune and metabolic gut response in broilers fed β-mannanase in β-mannancontaining diets. Poult Sci. 2017; 96: 4307-3216.
- Balasubramanian B, Ingale SL, Park JH, Rathi PC, Shanmugam S. et al. Inclusion of dietary β-mannanase improves performance and ileal digestibility and reduces ileal digesta viscosity of broilers fed corn-soybean meal based diet. Poult Sci. 2018; 97: 3097-3101.
- Jackson ME, Anderson DM, Hsiao HY, Mathis GF, Fodge DW. Beneficial effect of β-mannanase feed enzyme on performance of chicks challenged with Eimeria sp. And Clostridium perfringens. Av Dis. 2003; 47: 759-763.
- Pettey LA, Carter SD, Senne BW, Shriver JA. Effects of beta-mannanase addition to corn-soybean meal diets on growth performance, carcass traits, and nutrient digestibility of weanling and growing-finishing pigs. J Anim Sci. 2002; 80: 1012-1019.
- Mok CH, Lee JH, Kim BG. Effects of exogenous phytase and β-mannanase on ileal and total tract digestibility of energy and nutrient in palm kernel expeller-containing diets fed to growing pigs. Anim Feed Sci Technol. 2013; 186: 209-213.
- Upadhaya SD, Park JW, Lee JH, Kim IH. Ileal digestibility of nutrients and amino acids in low quality soybean meal sources treated with β-mannanase for growing pigs. Animal. 2016; 10: 1148-1154.
- Jeon SM, Hosseindoust A, Choi YH, Kim MJ, Kim KY, et al. Comparative standardized ileal amino acid digestibility and metabolizable energy contents of main feed ingredients for growing pigs when adding β-mannanase. Anim Nutr. 2019; 5: 359-365.
- Lv JN, Chen YQ, Guo XJ, Piao XS, Cao YH, et al. Effects of supplementation of β-mannanase in corn-soybean meal diets on performance and nutrient digestibility in growing pigs. Asian-Aust J Anim Sci. 2013; 26: 579-587.
- Jo JK, Ingale SL, Kim JS, Kim YW, Kim KH, et al. Effects of exogenous enzyme supplementation to corn- and soybean meal-based or complex diets on growth performance, nutrient digestibility, and blood metabolites in growing pigs. J Anim Sci. 2012 ; 90: 3041-3048.
- Vangroenweghe F, Suls L, Van Driessche E, Maes D, De Graef E. Health advantages of transition to batch management system in farrow-to-finish pig herds. Vet Med. 2012; 57: 83-91.
- Williams MP, Brown B, Rao S, Lee JT. Evaluation of beta-mannanase and nonstarch polysaccharide-degrading enzyme inclusion separately or intermittently in reduced energy diets fed to male broilers on performance and carcass yield. J Appl Poult Sci. 2014; 23: 715-723.