
Review Article
Austin Virol and Retrovirology. 2016; 3(1): 1020.
A Review on Rift Valley Fever on Animal, Human Health and its Impact on Live Stock Marketing
Kasye M, Teshome D*, Abiye A and Eshetu A
School of Veterinary Medicine, Wollo University, Ethiopia
*Corresponding author: Daniel Teshome Gebeyehu, School of Veterinary Medicine, Wollo University, Dessie, Ethiopia
Received: May 20, 2016; Accepted: June 23, 2016; Published: June 28, 2016
Abstract
Rift Valley Fever (RVF) is an acute, mosquito-borne viral disease that has significant global threat to livestock marketing and on human health. The disease is caused by a virus of the genus Phlebovirus of family Bunyaviridae, a group of enveloped single stranded RNA viruses. A review of RVF was made with the objective of organizing information on the epidemiology and management of rift valley fever, and on its economic impacts related to livestock marketing. The disease is mostly confined in Africa but it also occurs in other parts like Saudi Arabia and Yemen. Even though clinical disease has never been occurred in Ethiopia, serological tests are gotten IgM positive for RVF. The transmission of RVF is primarily by the bites of the mosquitoes of several species. Man acquires the infection from the infected animals and insect bites. Diagnosis is confirmed by PCR, isolation of virus, demonstration of antibodies in the sera and histopathology of the liver. Immunization of animals, destruction of mosquitoes and restriction on the movement of animals during epizootic can help in the control of RVF.
Keywords: Economic impact; Rift valley fever; Vaccines; Vectors; Virus
Introduction
The livestock sub-sector plays a vital role in national economy of many developed and developing countries. It profits the national gross domestic economy through benefits from cattle rearing, exporting of live animals and hide as well as skin; which all those earn huge amount of money for that country. However, many countries are not self-sufficient in livestock productions while livestock still remains an integral part of their national economy. The most important contribution and value of livestock’s are: source of food, drought power, social and cultural assets, source of income and means of transportation. The proportion of livestock in Ethiopia remained the largest figure in Africa until recent time but levels of production are one of the lowest [1].
The economic benefit derived from the livestock sector in Ethiopia is not commensurate with the potentials and the sub sector remained untapped [2]. Productivity of animals is poor; factors for the poor productivity of livestock in Ethiopia include: disease, poor nutrition, unimproved genotypes, inappropriate management, socio economic and institutional constraints [1]. The widely prevalent livestock diseases are major constraints to livestock exports. Livestock exports from Ethiopia are jeopardized by repeated bans in particular from the countries in Arabian Peninsula as carrying the risk of introducing number of trans-boundary livestock disease [2].
RVF is an acute febrile arthropod born viral diseases of sheep, goats, cattle as well as humans and presents in most countries of sub Saharan Africa. As a current threat of bioterrorism, it could appear in other parts of the world [3].
RVF was first reported in an outbreak of abortion and death in exotic wool sheep as well as illness in humans that occurred in the rift valley of Kenya after heavy rain fall in 1930-31. Out breaks since occurred in the high lands of Kenya at irregular intervals of 3-15 years [4]. But it exists and occurs as epizootics through sub Saharan Africa with recent extension into Egypt and Madagascar, Mauritania and most recent expansion to the Arabian Peninsula [5].
The most recent epizootics in East Africa region was in 1997- 1998 in the dried areas of North East Kenya and South West Somalia after a heavy El Nino associated rains. This cause’s human death and some livestock lose, particularly of camels, but more significantly disruption to livestock exportation to the Middle East from the Horn of Africa [6].
The epidemic of RVF in Horn of Africa in 1997/8 stimulated many countries, but most importantly the kingdom of Saud Arabia as the major trading partner, to instigate a ban on livestock imports from the region [2].
In Ethiopia during the same period, the heavy rain fall and attendant flooding affected Southern and South Eastern parts of the country bordering Somalia and Kenya. Veterinarian Field investigations carried out in Somalia region and Borena zone in 1998 have observed high level of usual abortion among livestock. Out of the samples collected two sera from small ruminants from Mustahil, just near the border with Somalia were found IgM positive to RVF [2].
The disease is currently an economical concern because of the cost associated with preventive measures in endemic areas, monitoring for introduction of disease in neighboring unaffected areas, and trade restriction on import and export to and from countries [7]. To understand RVF, make prevention and to be ready to take action in controlling, it is important to know the nature and epidemiology of the disease.
Therefore, the objectives of this seminar paper are, to review:
- Epidemiology and management of rift valley fever, and
- Economic impacts of rift valley fever related to livestock marketing.
- Sub Saharan countries and their trade partners should collaborate and consider cost effectiveness analysis for planning and monitoring of rift valley fever to benefit the most out of the livestock industry.
- Cyclical occurrence should be considered while planning surveillance program of rift valley fever.
- Awgchew K. The state of Ethiopian farm animal’s genetic resource country report, news letter number 10. Ethiopian Society Of state of animal production. Addis Ababa, Ethiopia. 2004.
- Wondosen F. Influence of animal disease and sanitary of regulation of livestock trade and case of export restriction. 10th Edn. Addis Ababa: Ethiopian society of state of animal production. 2003; 1-56.
- Smith P. Large animal internal medicine. 4th Edn. Mousy Elsevier, Davis, California. 2009; 919.
- Swanepoel R. Rift Valley fever. Infectious disease of livestock with special reference to South Africa: Cape Town. Oxford University Press. 2004; 1: 687- 717.
- Radostitis OM, Blood DC, Gay CC. Veterinary medicine, text book of the disease of cattle, sheep, pig, goats and horse.10th Edn. Spain: Saunders. 2007; 1205-1206.
- FAO. Rift valley fever threatens livelihood in horn of Africa: Empress Transboundary animal disease Bulletin Number 15. 2000.
- FAO. Rift valley fever threatens livelihoods in the horn of Africa. In: Empress trans-boundary animal disease Bulletin Number 16. 2001.
- Kahen CM. The Merck Veterinary Manual. 9th Edn. White House Station, New Jersey, USA. 2005; 617-619.
- Davis FG, Martin V, Clarke N. Recognizing Rift Valley Fever. FAO Animal Health Manual Number 17. 2003; 1-45.
- OIE. Manual of diagnostic tests and Vaccines for Terrestrial Animals. Office International des Epizootic Publication Paris, France. 2004.
- Joshi V. Textbook of Veterinary Public Health. 1st Edn. New Delhi: Indian Council of Agricultural Research, India. 2004; 317-319.
- Martin V, De Simone L, Lubroth J, Ceccato P, Chevalier V. Perspectives on using remotely sensed imagery in predictive veterinary epidemiology and Global Early Warning Systems. Geospatial Health. 2007; 2: 3-14.
- Chevalier V, Rakotondrafara T, Jourdan M, Heraud J, Andriamanivo H, Durand B, et al. An unexpected recurrent transmission of Rift Valley Fever virus in cattle in a temperate and mountainous area of Madagascar. Plos Neglected Tropical Diseases. 2011; 1-9.
- Nicolas G, Durand B, Duboz R, Rakotondravao R, Chevalier V. Description and analysis of the cattle trade network in the Madagascar highlands: potential role in the diffusion of Rift Valley fever virus. Acta Trop. 2013; 126: 19-27.
- Pal M. Zoonosis. 2nd Edn. New Delhi: Satyam. 2007; 115-118.
- Gerdes GH. Rift Valley fever. Rev Sci Tech. 2004; 23: 613-623.
- Quinn PJ, Carter ME, Markey BK, Carter GR. Clinical microbiology. 2nd Edn. Spain: Mosby. 2002; 399.
- Edward P, Maclachean N, Dubovi J. Ferner’s veterinary virology. 4th Edn. London: Elsevier. 2011; 376-380.
- WHO. Rift Valley Fever Fact Sheet No. 207. 2007.
- Ikegami T, Makino S, Nipp T. The pathogenesis of Rift Valley fever Virus. Infectious Disease of Livestock (b). 2nd Edn. Cape Town, Southern Africa: Oxford University Press. 2011; 1037-1070.
- USDA. Rift Valley fever diagnosis and vaccine development and evaluation. 2011.
- Hartley DM, Rinderknecht JL, Nipp TL, Clarke NP, Snowder GD. National Center for Foreign Animal and Zoonotic Disease Defense Advisory Group on Rift Valley Fever. Potential effects of Rift Valley fever in the United States. Emerg Infect Dis. 2011; 17: e1.
- Davies FG, Martin V. Recognizing Rift Valley Fever. Vet Ital. 2006; 42: 31-53.
- FAO. Preparation of Rift Valley Fever Contingency plans: Food and agricultural organization animal health manual number 6. Rome. 2003.
- Ochei J, Markey K, Akochatkar K. Medical laboratory science theory and practice. Tata McGraw-Hill publishing company limited. New Delhi. 2000; 880.
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals: Journal of Office International des Epizootic. 2008; 2: 56-61.
- WHO. Rift Valley Fever Fact Sheet No. 77. 2009.
- WHO. Rift Valley Fever Fact Sheet No. 36. 2008.
- FAO. Terrestrial Animal Health Code. Food and agricultural organization. USA Contributions to Epidemiology and Biostatics. 2005; 3: 178-190.
- Seifert SH. Tropical animal. 2nd Edn. Netherland: Klumer. 1996; 247.
- Empres M. Rift Valley Fever in Eastern Africa. Trans-boundary Animal Diseases Bulletin Issue Number 30. 2008.
- FAO. Manual on the recognition of rift valley fever: Food and agricultural organization animal health manual number 8. Rome. 2002.
- NICD. Rift valley fever diagnosis and prevention methods for Terrestrial Animal. National Investigation for Communicable Diseases, Switzerland, Geneva. 2011; 82.
- Bouloy M, Flick R. Reverse genetics technology for Rift Valley fever virus: Current and Future applications for the development of therapeutics and vaccines. Antiviral Research. 2009; 84:101-118.
- FAO. Preparation of Rift Valley fever contingency plans: Food and agricultural organization animal health manual number 15. Rome. 2004.
- Pratt N, Bonnet P, Jabbar M, Ehui S, deHaan C. Benefits and Costs of Compliance in Sanitary Regulations in Livestock Markets: The Case of Rift Valley Fever in Ethiopia. Paper presented at the 7th Annual Conference on Global Economic Analysis, Washington DC. 2004; 17-19.
- Aklilu Y. Livestock Marketing in Kenya and Ethiopia: A Review of Policies and Practice Pastoral Areas Coordination. Analysis and Policy Support Project. Feinstein International Center. Addis Ababa. 2008; 203.
- USAID. Multi-sectoral interventions in pastoralist communities in Horn of Africa. 2005.
- Bird B, Ksiazek S, Nichol T, Maclachlan N. Rift Valley fever virus: Journal of American Veterinary Medicine Association. 2009; 234: 883-893.
- Anonymous G. Brief report on the impact of Rift Valley fever in the Horn of Africa. 2007.
- Rich KM, Perry BD. The economic and poverty impacts of animal diseases in developing countries: new roles, new demands for economics and epidemiology. Prev Vet Med. 2011; 101: 133-147.
- Handlos M. Assessment of the estimated costs of past disease outbreaks in Yemen, Rain fed Agriculture and Livestock Project: International expertise service for the General Directorate of Animal Resources, Yemen. Sana’a and Vientiane, Yemen. IDA CR. No. 4220 Yemen. 2009.
- Soumare B, Thys D, Berkvens A, Hashi V, Huylenbroeck G. Effects of livestock import bans imposed by Saudi Arabia on Somaliland for sanitary reasons related to Rift Valley fever. Journal of Outlook Agricultural Research. 2006; 35: 19-24.
- Bonnet P, Tibbo M, Workalemahu A, Gau M. Rift Valley fever and emerging threat to livestock trade and food security in the Horn of Africa. Ethiopian Society of Animal Production (ESAP). Addis Ababa, Ethiopia. 2001; 379-404.
- Sindato C, Karimuribo E, Mboera LE. The epidemiology and socio-economic impact of rift valley fever epidemics in Tanzania: a review. Tanzan J Health Res. 2011; 13: 305-318.
- Abdo-Salem S, Tran A, Grosbois V, Gerbier G, Al-Qadasi M, Saeed K, et al. Can environmental and socioeconomic factors explain the recent emergence of Rift Valley fever in Yemen. Vector borne zoonotic disease, 2011; 11: 773- 779.
- Zinsstag J, Schelling E, Roth F, Bonfoh B, de Savigny D, Tanner M. Human benefits of animal interventions for zoonosis control. Emerg Infect Dis. 2007; 13: 527-531.
- Whittington RJ, Chong R. Global trade in ornamental fish from an Australian perspective: the case for revised import risk analysis and management strategies. Prev Vet Med. 2007; 81: 92-116.
- Health Code. World Organisation for Animal Health, Paris, France.
Rift Valley Fever
RVF is an acute mosquito born viral disease mainly affecting ruminant animals and humans. It can cause abortion in pregnant animals and a high mortality in young animals. In human RVF causes a sever influenza like illness, with occasionally more series hemorrhagic complications and death. Over its range, it causes major epidemics at irregular intervals of 5-35 years [6].
Etiology
RVF is caused by RVF virus which belongs to the family Bunyaviridae and the genus Phlebovirus. These are spherical virions with diameter of 80-120 nanometers and a host cell derived, bi lipid layer envelop through which virus coded glycoprotein spikes project [8]. This single stranded Ribose Nucleic Acid (RNA) virus has a lipid envelope and two surface glycoproteins, G1 and G2. The genome has three segments: L (Large), M (Medium) and S (Small). RVF virus replicates in the mosquitoes and in the vertebrate animals. The liver, spleen and brain are the major sites of viral replication. The Virus is resistant in alkaline environments but inactivated at pH <6.8.The virus can be inactivated by disinfectants such as calcium hypochlorite, sodium hypochlorite and acetic acid; and be maintained for 8 years when stored below 0°C [9].
Epidemiology
Distribution and occurrence: Although RVF is still confined to the African continent it has great potentials for spread to other countries [5]. The disease is endemic in Southern and Tropical regions of many Eastern Africa countries, although an epidemic was reported in 2002 Saudi Arabia and Yemen. The main occurrence of the disease epizootics was observed in Eastern and Central Africa. The first thoroughly investigated epizootic begins in 1930 [8].
The incidence of RVF peaks in late summer. The virus is spread epidemically by many species of mosquitoes. Since its first outbreak among the sheep in 1931, the disease has been reported in several other species of animals and man. Countries with endemic disease and substantial outbreaks of RVF include Egypt, Gambia, Kenya, Madagascar, Mauritania, Mozambique, Namibia, Saudi Arabia, Senegal, South Africa, Sudan, Yemen, Zambia and imbabwe [10].
The cyclic epidemics have occurred at 5-20 years intervals in drier areas. In the periods between epidemics, the virus is believed to be dormant in eggs of the mosquitoes. Many countries like Angola, Botswana, Burkina Faso, Cameroon, Chad, Congo, Ethiopia, Gabon, Guinea, Malawi, Mali, Niger, Nigeria, Somalia, Tanzania and Uganda are known to have some cases, periodic isolation of virus, or serologic evidence of RVF [11].
Rift Valley fever has been reported in four ecological systems: Dambo areas, semi-arid areas, irrigated areas and temperate and mountainous areas (Figure 1).
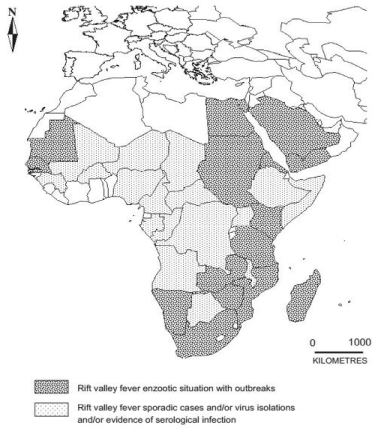
Figure 1: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
Dambo areas these are shallow depressions, often located near rivers, which fill with water during the rainy season. In Dambo areas, a correlation between heavy rainfall and RVF epidemics has been demonstrated. Transmission from one mosquito generation to another, i.e. vertical transmission, has been demonstrated in Aedes in these ecosystems. In addition, the virus may survive in Aedes eggs, which are resistant to desiccation in the environment over a long period of time during inter-epidemic dry/cold periods. Owing these two mechanisms, and to extreme rainy events particularly during El Nino phenomena, the disease may re-emerge every 5-15 years, with only a few infections during the inter-epizootic period [12].
Semi-arid areas- these areas which RVF has been reported, are characterized by temporary water points, such as found in northern Senegal or Mauritania. However, in these areas the virus persistence remains unclear. It could be related to the survival of the virus in Aedes mosquitoes, as demonstrated in East Africa or to the regular introduction of the virus by nomadic herds coming from neighboring endemic areas [13].
Irrigated areas –are areas where RVF occurs including the Nile Delta (Egypt) and the Senegal River valley (Senegal, Mauritania), where permanent water bodies favor the development of Culex populations, and thus yearlong viral transmission [13].
Temperate and mountainous areas- such as those found in Madagascar, favors transmission of RVF virus by local vectors associated with specific cattle trade practices [14]. In some of the ecosystems in South Africa and Zimbabwe virus circulation maintained between mosquito vectors and wild ruminants in sylvatic cycles [13].
Host range and susceptibility: Natural infection due to RVF virus has been recorded in antelope, buffalo, camel, cattle, goat, monkey, rodents, and sheep besides man [15]. Significant mortality and morbidity due to RVF have been reported in sheep, cattle and man [8]. Several species of domestic, pet, farm and laboratory animals are susceptible to RVF virus. The kids, lambs, puppies, kittens, hamsters and mice are highly susceptible to RVF virus. Amphibians and reptiles are resistant to RVF virus [16].
Morbidity and mortality: Young animals, such as lambs, kids, puppies, and kittens are considered as extremely susceptible with mortality of 70-100 percent. Sheep and calves are considered as highly susceptible with mortality rates between 20-70 percent. Adult cattle, goats, buffaloes, and humans are considered as moderately susceptible and mortality rate is typically less than ten percent; for humans the case fatality rate is typically less than one percent. Equines, pigs, dogs and cats are categorized as resistant and infection is unapparent (Table 1) [10].
Mortality >70%
Mortality 10-70%
Sever disease with low fatality rate (<10%)
Antibody production
Not susceptible
Lamb Kid
Puppy kitten
Mouse
Rat
Sheep Calf
Some rodent
Human Cattle Goat
African buffalo
Asian buffalo
Monkey
Camel Horse
Cat Dog
Swine Donkey
Rabbit
Bird reptiles Amphibians
Table 1: Species susceptibility of rift valley fever virus. Source: (OIE, [26]).
Source of infection: A pronounced viremia occurs in infected animals for about a week and facilitates the spread of the disease of biting insects. During viremic period blood, tissue of affected animals, aborted fetus and fomites are source of infection [17].
Mode of transmission: It was founded that the virus is transmitted transovarially among flood water Aedes species mosquitoes. The virus survives for long periods in mosquito eggs laid at the edge of usual dry depression, called dambos, which are common through grassy plateau regions. When the rain comes and these dambos flood, the egg hatch and affected mosquitoes emerge and infect nearby wild and domestic animals. Direct and indirect transmission can occur via aerosol, contact with infected placenta or aborted fetus, fomites or mechanical transport on the mouth part of flies [17].
Animals and man get infection following the bites of many species of mosquitoes. The virus survives for very long periods in the mosquito eggs. Cattle and sheep are primary amplifiers of the virus. The capacity of RVF virus to transmit without the involvement of an arthropod vectors raises concern over the possibility of the virus for its importation in to non-enzootic areas through contaminated materials, animal products, viremic humans or no livestock animal species [18]. Low concentration of RVF virus in the milk of sick animal may pose health risks to man if the milk is consumed raw or unpasteurized [19]. Humans have the potential to introduce RVF virus through mosquitoes bite to animals in uninfected areas [8].
Risk factors: The incidence of the disease varies with size of the vector population and it is greatest in season of heavy rainfall. This allows the vector population to breed in surface water in normally dry areas [5].
Most indigenous livestock species in Africa demonstrate a high level of resistance to the disease. A high degree of herd immunity arises in locations where infections are most intense and it was one of the factors which contribute to the abatement of enzootics. Because of the massive immunity produced in recovered animals which is also transferred passively with the colostrums from the dam to calf and from the ewe to lambs, enzootics appear only in interval of 4-7 years [4].
Clinical manifestations and diagnosis of rift valley fever
Clinical manifestations of rift valley fever
Pathogenesis: RVF virus replicates rapidly and to very higher titer in target tissues after entry by mosquitoes bite, percutaneous injury or through the oropharynx through aerosols [18].
After infection the virus spread from the initial site of replication to critical organs such as the spleen, liver and brain which are either damaged by the pathogenic effects of the virus or immunopathological mechanisms, else there is recovery mediated by nonspecific and specific host response. The virus is conveyed from the inoculation site by lymphatic drainages to regulate lymph nodes where there is replication and spin over into the circulation which leads to viremia and systemic infections [20].
Clinical signs: The incubation period varies from 1 to 6 days. The incubation period is 12-72 hours for newborn lambs, 24-72 hours in adult sheep, goats, and cattle, and 3-6 days in humans [21]. RVF is characterized by high abortion rates and high mortality in neonates usually occurring after periods of heavy rainfall and clinical signs include:
Cattle: Calves experience fever (40-41°C), in appetence, weakness, depression, diarrhea, and jaundice. Adults often experience unapparent infection; but fever lasting 24-96 hours, dull coat, lacrimation, nasal discharge, excessive salivation, anorexia, weakness, bloody diarrhea, low milk yield, and high abortion rates in pregnant cows are common [22].
Sheep and Goats: Newborn lambs (less than 2 weeks of age) experience biphasic fever (40-41°C), anorexia, weakness, abdominal pain, rapid respiration, and death within 24-36 hours. Lambs (over 2 weeks of age), adult sheep and goats experience fever lasting 24- 96 hours, anorexia, weakness, depression, increased respiratory rate, vomiting, bloody diarrhea, mucopurulent nasal discharge, jaundice and abortion rates approaching 100 percent [22].
Pathologic lesions: Hepatic necrosis is the primary lesion observed in RVF. In aborted fetuses and neonatal animals, particularly the lambs and calves, the liver is soft, enlarged, friable and yellowish brown to dark in color. In addition, the edema and hemorrhages in the wall of gall bladder, hemorrhagic enteritis, enlarged edematous peripheral and visceral lymph nodes, widespread cutanous hemorrhages, accumulation of blood stained fluids in the body cavities and extensive subcutaneous and serosal hemorrhages are also observed. The rapid decaying of the carcass may be a consequence of the severe liver damage. In lambs there is a focal to diffuse coagulative necrosis of hepatocytes in the affected liver. Intramuscular inclusion bodies are also noticed [23].
Clinical pathology: Sever leucopenia, high blood levels of enzymes like; glutamic dehydrognase (GLDH), associated with liver damage and thrombocytopenia are common findings (Figure 2) [24].
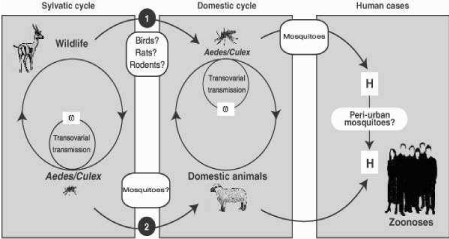
Figure 2: Rift valley fever transmission cycle. (Source: FAO [24]).
Diagnosis of rift valley fever
Clinical diagnosis: Affected animals show fever (40-42°C), anorexia, depression, weakness, mucopurulent nasal discharge, vomiting, jaundice and hemorrhagic diarrhea [4].
Differential diagnosis: Differential diagnoses in animals include: blue tongue, heart water disease, Nairobi sheep disease, ephemeral fever, brucellosis, Wessel borne disease, pest des petites ruminitis, foot and mouth disease. Nairobi sheep disease has no hepatitis and does not occur in new born lambs. In case of bluetongue no hepatitis and lesions on mouth and foot (coronitis) are common. Serous fluid in body cavities and neurological signs are common in heart water disease. In ephemeral fever there is recumbence (muscle weakness), rapid recovery and is not commonly occur in sheep and goats, while brucellosis does not occur in relation with heavy rain fall. Wessel borne disease is rare viral disease and less severe than RVF. Pest des petites ruminitis has high mortality in lambs while foot and mouth disease has neonatal mortality and abortions in small ruminants [16].
Laboratory diagnosis: There are several methods by which RVF virus can be diagnosed in clinical laboratories. These clinical laboratory methods include:
Direct methods: it is based on detection of viral particles; viral antigens or nucleic acid and detection of characteristic changes in tissues at site of infections. Histopathologically, examination of the liver of the affected animal reveals extensive hepatic necrosis which is the characteristic lesion in RVF [5].
Isolation and identification of causative agent: isolation of infected virus from appropriate specimen and its identification can establish diagnosis. Isolation of infecting virus in cell culture is most sensitive method of diagnosing viral disease [25].
The clinical specimens such as the liver, spleen, brain, lymph nodes, kidney, heart, and blood should be collected aseptically in sterile container for virus isolation. In case of an autolysed fetus, the brain is a good specimen to be submitted on ice to the laboratory for diagnosis. Several types of cell cultures such as Baby Hamster Kidney (BHK), African green monkey kidney (Vero), Chicken Embryo Reticulum (CER), or primary kidney and testis cell cultures of lambs and laboratory animals like mice and hamsters can be employed for the isolation of virus [8].
Serologic diagnosis: detection of specific viral antibody and their quantification at various stages of the disease is an important tool for the diagnosis of many viral diseases. Because of its broad geographic distribution and its explosive potential for invading new areas where livestock husbandry is extensive, the laboratory confirmation of the presence of RVF virus is treated as a diagnostic emergency [26]. Two sera samples at an interval of 30 days should be obtained to demonstrate antibodies against RVF by Enzyme Linked Immune Sorbent Assay (ELISA), Agar Gel Immune Diffusion (AGID), Hemagglutination (HI) and virus neutralization methods [10].
The virus antigen can be detected by Reverse Transcriptase Polymerase Chain Reaction (RT-PCR). It is a very specific and sensitive molecular tool for the diagnosis of RVF in the early phase of disease. PCR used for rapid diagnosis for antigen detection and used to detect RVF virus in mosquito pools. RT-PCR followed by sequencing of the nucleocapsid protein-coding region has been used in phylogenetic analysis [27].
Virus neutralization (the prescribed test for international trade): Cannot differentiate presence of antibodies of naturally infected animals from animals vaccinated with RVF vaccine; detects antibodies against RVF virus in the serum of a variety of species highly specific and will record the earliest response. These tests can only be performed with live virus; thus not recommended for use outside endemic areas or in laboratories without appropriate biosecurity facilities and vaccinated personnel [26].
ELISA: can be performed with inactivated antigen and can therefore be used in RVF-free countries. Cross-reactions may occur between RVF virus and other Phleboviruses. Use of inactivated whole virus or mouse liver antigens has recently been replaced by recombinant nucleocapsid protein as antigen. IgM capture ELISA allows diagnosis of a recent infection [28]. Hemagglutination inhibition can be performed with inactivated antigen and can therefore be used in RVF free countries. It employed with great confidence in non-endemic areas [27].
Field diagnosis: RVF should be suspected when abnormally heavy rains fall is followed by the wide spread occurrence of abortion and mortality among new born animals characterized by necrotic hepatitis and when hemorrhages and influenza like disease are seen in people handling animals or their products [8]. The field veterinarian can suspect RVF if he/she encounters high abortion rates possibly approaching 100 percent among the cows and ewes; very high mortality approximately 100 per cent in calves and lambs of less than 7 days of age, extensive liver lesions in aborted fetuses and neonatal animals, an influenza like disease in man particularly in individual associated with livestock and occurrence of disease during a period of high insect activity [10].
Control and prevention
Vaccinations: There is no specific treatment for RVF. However, two vaccines are available and are commonly used for control of RVF in endemic countries: a live attenuated vaccine and a formalin inactivated vaccine. The live attenuated Smith burn vaccine induces lifelong immunity in sheep and goats. The Smith burn vaccine has a potential for reversion, so it is not recommended for widespread use in non-endemic countries or during outbreaks. The inactivated vaccine does not confer long term immunity and thus requires booster vaccination and annual revaccination for continued protection against infection. The inactivated vaccine is recommended for use in pregnant animals and in RVF free countries experiencing outbreaks [19].
RVF can be controlled by vaccination of all susceptible animals to prevent infection of amplifying hosts and thus infection of vectors. Use of insecticides to kill mosquitoes and ban on export of livestock during RVF epizootic periods is also recommended [15].
Vector control: Mosquitoes are the most important way that RVF is spread. It is only the female mosquito that feeds on blood as she needs the protein to produce eggs. Mosquitoes will lay their eggs on or near the edge of water. The mosquito eggs will hatch into larvae (also known as wigglers) which turn into pupae (also known as tumblers). The larvae and the pupae need to live in water to survive. The pupae will change into adult mosquitoes [29].
Control of mosquito egg laying sites: mosquitoes can lay their eggs any place that can hold water. This includes: ponds, old tires, tarps, tree holes, bird baths and flower pots. This is the best way to control mosquitoes since they lay eggs in specific areas and these areas can be managed [29].
Control of mosquito larvae (wigglers): mosquito larvae need to live in water to survive. They can be found in any amount of standing water including ponds, old tires, tarps and bird baths. Since mosquito larvae remain in the same water where they hatched from eggs, control of this stage focuses on continued management of mosquito egg laying areas [29].
Control of mosquito adults: this is the least effective way to control mosquitoes. Attempting to control adult mosquitoes can be difficult and costy. Control of adult mosquitoes focuses on the use of pesticides [29].
Reducing host exposure to biting Aedes: The movement of stock from low lying areas to well drain and winds wept pastures of higher altitude, or confinement of animals to mosquito proof sheds may be measures of management to reduce the incidence of RVF. The breeding management may also take in to account the seasonal activities of vectors and prevents the lambing and or calving season during the rainy season [30].
Prevention of introduction of rift valley fever: Movement control refers to activities regulating the movement of people, animals, animal products, vehicles, and equipment in an area subject to certain criteria. Movement control is accomplished through a permit system that allows entities to make necessary movements without creating an unacceptable risk of disease spread [31].
Quarantine refers to imposing restrictions on entering or leaving a premise, area or region where disease exists or is suspected. Quarantine stops the movement of infected animals, contaminated animal products, and fomites from infected, contact, and suspect premises [31].
Infection can be introduced in to an area free of RVF by infected animals, animal products and insects (Aedes) [32].
Impacts of Rift Valley Fever on Human Health
RVFV is considered as a major zoonotic threat which is classified as a high consequence pathogen with potential for international spread list “A” disease, because of its potential to cause severe disease in both animals and man during outbreaks [26].
Risk group
Occupational groups such as herders, farmers and farm workers, abattoir workers and veterinarians/animal health workers are at especially high risk of infection [15].
How human acquire infection?
Direct or indirect contact with the blood or tissues of infected animals can transmit the virus. This may include: handling of animal tissue during slaughtering, butchering or skinning of animals, assisting with animal births, conducting veterinary surgical procedures or disposal of carcasses/fetuses. Less common modes of transmission include: inoculation, for example via a wound from an infected knife or needle stick injuries or contact with broken skin, inhalation of aerosols produced during the slaughter/necropsy of infected animals, bites of infected mosquitoes (most commonly Aedes) and consuming raw (unpasteurized or uncooked) milk from infected animals. No human to human transmission has ever been documented [26].
Clinical signs
RVF presents in humans as influenza-like syndrome characterized by fever (37.8-40°C), headache, myalgia, weakness, nausea and light sensitivity. Complications can arise and result in retinopathy, blindness, meningoencephalitis, hemorrhagic syndrome with jaundice, petechiae and death [33]. The illness may be mild or sever.
Mild illness: The incubation period (interval from infection to onset of symptoms) for RVF varies from two to six days. Clinically, it presents as a fever with flu-like symptoms (including myalgia, arthralgia and headache). Some patients may also develop neck stiffness, sensitivity to light (photophobia), loss of appetite and vomiting; in such patients the clinical presentation may be mistaken for meningitis. Symptoms of RVF usually last from four to seven days, after which the immune response becomes detectable with the appearance of antibodies and the virus gradually disappears from the blood [33].
Severe illness: A small percentage of patients develop a much more severe form of the disease, which can manifest as one or more of ocular disease (retinitis), meningoencephalitis, hepatitis and renal failure [33].
All researches and diagnostic procedures with RVF virus are restricted to certain national laboratories. The virus is a biosafety level three pathogen, and must be handled in the laboratories under strict biosafety cabinet to prevent human exposures. As RVF is a zoonotic disease, all precautions should be taken to protect the health of the persons engaged in livestock industry [18].
Impacts of Rift Valley Fever on Livestock Marketing
In Africa, pastoralism plays an important role in national economies. In particular, the export of livestock from the pastoral communities to the Middle East is of vital economic importance as millions of animals are exported each year, particularly during the religious festival periods.RVF virus is considered as a potential bioterrorism tool that could have direct (morbidity and death) and indirect (restriction in international trade) impact in countries that are free from the virus [34].
Rift valley fever as trans-boundary disease
Trans-boundary diseases are permanent threat for livestock keepers. They have major economic implications both through the private and public cost of outbreaks and through the cost of the measures taken at individual, collective and international levels in order to prevent or control infection and disease out breaks [35].
Trans-boundary diseases are diseases that are significant in economic trade and or food security importance for a considerable number of countries, which can easily spread to other countries and reach epidemic proportion; and where control or management, including exclusion requires cooperation between several countries [31].
As a list ‘A’ disease among the Office International Des Epizootics (OIE) classification of contagious diseases being threats for international economy, RVF is a major stake for the establishment of nontariff barriers. The ban on livestock imports from the Horn of Africa was apparently imposed for public health reasons because of concern that slaughter of RVF virus infected livestock could result in disease transmission to people. OIE regulations refer two types of country status with regard to RVF: free and non free countries, given the present status and the animal health situation in Ethiopia, the possibility of Ethiopia being declared free from RVF is considered a major effort not attainable in the near future [35].
Rift valley fever in Ethiopia
In the Horn of Africa, the Somali region of Ethiopia is one of the most active livestock trading areas, and various sources estimate that60-80% of Somalia’s livestock exports originate from this region of Ethiopia through a largely informal cross border trade [36].
Following an outbreak of RVF in Kenya, the United Arab Emirates imposed an export ban on Ethiopia for about six months in 2007. A recent outbreak of RVF in East Africa has led to an export ban by Saudi Arabia and other Gulf countries on livestock products from Ethiopia. Until 1998, several million sheep and goats were exported every year to Saudi Arabia from ports in Somalia, correlating with the Hajji activities in Mecca. Pastoral populations in Ethiopia’s south Eastern lowlands depend heavily on livestock exports to Somalia for their livelihoods, most of which are exported to Saudi Arabia and other Gulf States. The trade has proceeded for many years, until an outbreak of RVF in the region of the Horn of Africa (Sudan, Kenya, Somalia, Eritrea, Ethiopia and Djibouti) prompted two consecutive bans by Saudi Arabian authorities in 1998 and 2000 [37].
RVF clinical disease has never been reported in Ethiopia.RVF was reported to OIE following positive serological tests but no clinical disease. The geographical localization of the country, associated with large commercial ruminant trade and pastoralist’s movement makes Ethiopia at risk for RVF occurrence [29].
In 2009/10 National Animal Health Diagnostic and Investigation Center (NAHDIC) in collaboration with regional veterinary laboratories has collected a total of 14,328 serum samples for RVF, foot and mouth disease, pest des petites ruminitis and brucellosis. The overall prevalence of the diseases was 0%, 11%, 57% and 0.4%, respectively [33].
Rift Valley fever is one of the most important diseases that affect the export of live animals and meat to prime markets in the Middle East countries. Since 1997/98, Ethiopia has faced a total of three bans as a result of epidemic situations of the disease in Kenya and Somalia. Although clinical cases of the disease have never been reported in Ethiopia, its geographical proximity to RVF endemic countries like Kenya, Sudan and Somalia, the nature of livestock movements across the international border and the ease with which infected mosquitoes can be moved longer distances by the help of wind can lead to the conclusion that Ethiopia will always be vulnerable to clinical RVF during the epizootic periods of the disease in East Africa [31].
The major parts of Ethiopian highlands do not favor vector survival and multiplication but the lowlands are seasonally flooded as a result of heavy rain in the highlands. These areas also border infected areas in neighboring countries. In line with this, Ethiopia had prepared RVF contingency and response plan in June 2008. The plan contains details of the resources needed in terms of personnel, equipment and other facilities that are required to tackle emergencies as a result of RVF as well as action plans for efficient and rapid development of both human and material resources for effective containment of the disease and elimination of infection. Based on the plan, NAHDIC and Regional veterinary laboratories are conducting annual surveillance programs against the disease in high risk areas of the country. This is intensively carried out when climatic forecasts indicate a possible multiplication and propagation of mosquitoes in the lowland parts of the country [33].
Economic impacts of rift valley fever
The economic impacts of RVF include death of animals and abortion, jeopardization of animal trade, devastating food security and cost of control. Pastoral communities relying on a livestock economy are highly vulnerable to the threat of disease to their livestock such as RVF [23].
Moreover in the context of the Horn of Africa, pastoralists who represent 15-20 million people in Djibouti, Eritrea, Ethiopia, Kenya, Somalia and Sudan have turned to a market integration and international trade orientation. This has led to new development opportunities but also to new economic threats, by increasing interdependence with the international economy [38].
The first reported direct socio-economic impact of RVF was on livestock producers due to high levels of mortality and morbidity in animals. This represents an important loss of stock, especially in young ruminants [39]. In addition, the disturbance on herd dynamics could result in production losses lasting several years or even several animal generations (long term effects). These effects are perceived over the long term and are subject to the combined influence of other economic mechanisms besides the strict herd dynamics [40].
The impact of RVF on producers will have repercussions along the livestock value chain (production and market activities) and its ancillary services. Cumulatively, the impact on other service providers within the livestock supply chain and other parts of the larger economy can be greater than the impact of RVF at the farm level [41]. The impacts may be short (<1 year) or long term (over 1 year) and qualitative (value chain restructuring) and/or quantitative (performances and socio-economic values). These impacts are partially due to changes in the value and quantity of animals on the market [36]. Beyond the livestock value chain, there may be spillover effects on other agricultural value chains (e.g. the importation of other agricultural products may be banned from the infected countries as well as non-agricultural sectors, such as transportation or tourism) [42].
RVF outbreaks may result in the enforcement of embargoes on the exportation of live animals and animal products, as imposed by international sanitary policies. Where the banned export sector has an important economic weight in national trade balance, the ban may significantly affect the national economy [43]. Hence, the successive RVF related trade bans could impact the public treasury, the exchange rate of national currency and the price of imported goods [44].
Livestock exports play a major role as a source of employment, income and foreign exchange [45]. The export bans thus lead to decreasing livestock prices and worsening terms of trade, which further undermine pastoralist’s purchasing power and livelihood. The impact on livestock marketing is more severe during the major public religious feasts. During these periods, the risk of RVF infection increases because of a high density of animals and the religious practices [46]. The zoonotic nature of RVF results loss of confidence by an importing country and trigger a long lasting embargo as well as major economic and social repercussions on all the sectors (livestock and other industries) [41].
Zoonosises, such as RVF are particular risk of developing and transition countries. The public health infrastructures are in limited settings and not sufficient to support and sustain routine infectious disease surveillance, prevention and control activities, especially when outbreaks are known to occur every 5-15 years [47-49].
Conclusion and Recommendations
Rift Valley fever is economically important disease. In addition to its impact on animal health the impact it results due to import and export restriction is significant particularly in those countries which livestock contributes great share in their economy. As rift valley fever needs insects, a mosquito, for its life cycle and transmission, its epidemics has cyclical occurrence. The disease affects different species of animals including humans. Immunization and vector control are the main strategies to reduce the incidence of RVF. It is considered as an occupational disease of livestock handlers, dairy farmers, abattoir workers and veterinarians.
Based on the above conclusions the following recommendations are forwarded:
References