
Case Report
Austin J Womens Health. 2015; 2(2): 1012.
Giant High-Grade Endometrial Stromal Sarcoma Myxoid Variant
Variant Torres-de la Roche LA¹*, Vrentas V², Henke RP³, Pozder E4, Larbig A5, Taperek-Mildner R5 and De Wilde RL5
¹Clinic for Gynecology, Obstetrics and Gynecological Oncology at Pius Hospital, University Hospital for Gynecology, Carl von Ossietzky University Medical School Oldenburg, Germany
²Clinic for Gynecology, Obstetrics and Gynecological Oncology at Pius Hospital, Oldenburg, Germany.
³Institute of Pathology, Oldenburg, Germany
4Clinic for Gynecology and Obstetrics of Carl von Ossietzky University Oldenburg, Germany
5Clinic for Gynecology, Obstetrics and Gynecological Oncology at Pius Hospital, Oldenburg, Germany
*Corresponding author: Torres-de la Roche LA, Clinic for Gynecology, Obstetrics and Gynecological Oncology at Pius Hospital, University Hospital for Gynecology, Carl von Ossietzky University Medical School Oldenburg, Germany
Received: July 24, 2014; Accepted: August 26, 2015; Published: August 28, 2015
Abstract
Introduction: High-grade endometrial stromal sarcoma is characterized by high Cyclin D1 expression and YWHAE-FAM22 genetic fusion. A rare case of myxoid high-grade endometrial stromal sarcoma with fertility-sparing surgery is described, adding to the evidence and better understanding one of the aggressive tumors of the uterine corpus.
Case Presentation: A 36-year old nulliparous woman with desire to have children, rapid abdominal growth, abdominal pain, and suspicion of leiomyoma underwent laparotomy, where a left intraligamentary tumor of 30cm, 8,5 kg size was removed and the posterior uterine wall reconstructed. Histological, immunohistochemical and genetic tests confirmed high grade endometrial stromal sarcoma, myxoid variant, with high cyclin D1 expression and YWHAE t (10;17) (q22;p13.3) rearrangements. Despite counselling, the patient refused complete hysterectomy.
Conclusion: Pre-surgical diagnosis of endometrial sarcomas remains difficult. The use of immunohisto chemistry and genetic profile testing permit description of new variants of these tumors, allowing for appropriate counselling and management of patients.
Keywords: Endometrial neoplasms; Sarcoma; Endometrial stromal tumors; Cyklin D1; Oncogene proteins; FusionYWHAE
Abbreviations
EST: Endometrial Stromal Tumors; LGESS: Low-Grade Endometrial Stromal Sarcoma; HGESS: High-Grade Endometrial Stromal Sarcoma; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; FISH: Fluorescence In Situ Hybridization
Introduction
From the group of uterine corpus mesenchymal tumors, leiomyosarcomas and Endometrial Stromal Tumors (EST) are the most common malignancies [1,2]. The European Sarcoma Network Working Group recommends use of the WHO 2014 classification of tumors of female reproductive organs, where EST are subdivided into Endometrial Stromal Nodules, Low-Grade Endometrial Stromal Sarcoma (LGESS), High-Grade Endometrial Stromal Sarcoma (HGESS) and Undifferentiated Uterine Sarcoma [1,3]. EST is frequent in older women, but 10 - 25 % of cases are premenopausal women between 42 - 58 years. Black women are more often affected than white. Other recognized risk factors are prior pelvic radiotherapy, prior administration of tamoxifen, and genetics [2,4]. Most women also have abnormal uterine bleeding and dysmenorrhea, but 25% of patients, especially young women, may be asymptomatic [1,2]. EST account for < 10 % of uterine mesenchymal tumors, and 0.2 % of all uterine malignancies [2]. Incidence is 1-2 cases per million women worldwide [4], and 1.32/100 000 women in Germany [3].
HGESS characteristically have a poor prognosis, with a median progression time between 7-23 months after primary treatment, and 5-year overall survival rate less than 57%. Furthermore, HGESS with YWHAE-FAMM22 rearrangements are more aggressive and have still shorter survival rates [5,6]. The case that we presented displayed none of these risk factors, was nearly a decade younger than the average and, like most patients, presented with uterine enlargement and pelvic pain, and was initially operated with a suspected leiomyoma.
Case Presentation
A 36-year old woman was referred to our center with a diagnosis of giant leiomyoma, a history of 4-month symptomatic abdominal pain, rapid abdominal growth, edema, weight loss and varicose veins on her left leg with associated chronic pain. She was nulliparous with regular menstrual cycles, and desire to have children. Two years before, she had hadright adnexectomy because of an ovarian cyst, right-sided hydronephrosis and secondary renal infarct. Upon physical examination, cachexia was apparent, with a palpable tumor from the xiphoid process to the pubis, and a Grade 2 venous insufficiency in the left leg (Figure 1,2).
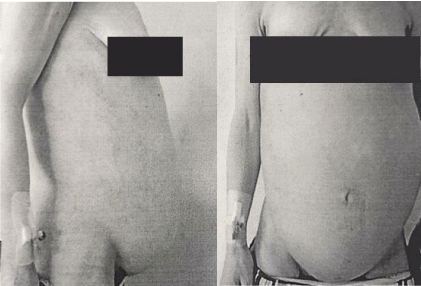
Figure 1 and 2: Physical appearance of patient before surgery.
Source: Patient´s clinical chart.
A giant non-homogenous tumor clinically presenting as a leiomyoma, measuring 30cm from the xiphoid process to the pubis was confirmed by ultrasonography, with all surrounding organs displaced and compressed. Computed Tomography (CT) imaging showed a presumable leiomyoma measuring 30Lx27Tx17AP cm; accompanying ascites; right-sided hydronephrotic and polycystic kidney with ureteral diameter of 9 mm; and normal abdominal and pelvic lymph nodes. There was no evidence of metastasis (Figure 3,4). Doppler showed pelvic and femoral vein and nerve compression in the left leg. Cystoscopy and pulmonary thoracic X-ray were normal. Blood test showed chronic anemia (Hb= 8.9 mg7dl); high levels of Ca 12-5 (148.8 IU/ml) and Ca 15-3 (37.5 IU/ml); VEG (68 mm); and thyroidal dysfunction (TSH= 7.74μIE/ml, T4=1. 44 ng/dl).
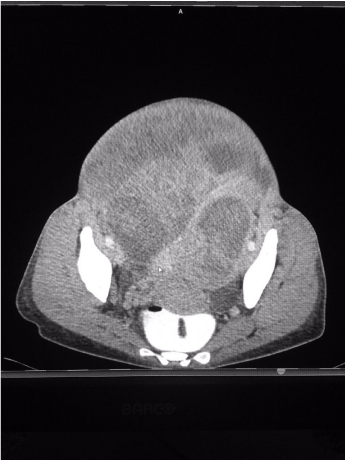
Figure 3: CT Cross-sectional imaging of tumor.
Source: Patient´s clinical chart.
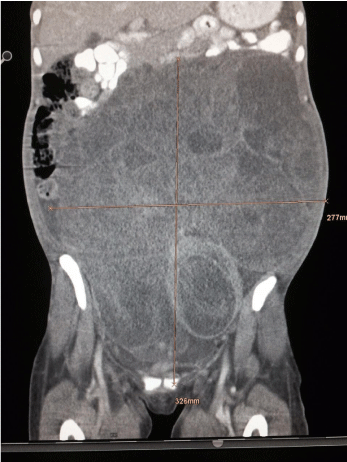
Figure 4: CT Longitudinal-imaging of tumor.
Source: Patient´s clinical chart.
The patient underwent laparotomy, revealing a left intraligamentary tumor resembling a large leiomyoma occupying the entire abdominal cavity reaching the xiphoid process, with the left Fallopian tube stretching over the tumor, the fimbria are obliterated.
The uterine corpus, cervix and appendix were normal. Ascites was present, with widespread peritoneal mesothelial reaction, multiple adhesions between the tumor and bowels, and several serous cysts (mean=1 cm) in the vesicouterine excavation. Additionally, stage IV endometriosis was found, with multiple pelvic endometriotic foci on the sigmoid colon and left ovary (mean=0,5cm), with complete Douglas pouch obliteration (Figures 5-8).
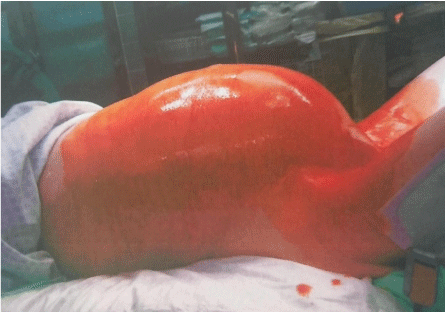
Figure 5: Lateral view of abdomen.
Source: Patient´s clinical chart.
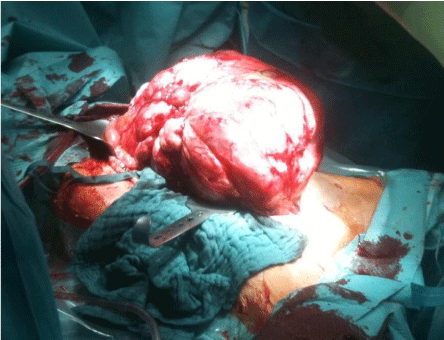
Figure 6: Intraoperative view of tumor.
Source: Patient´s clinical chart.
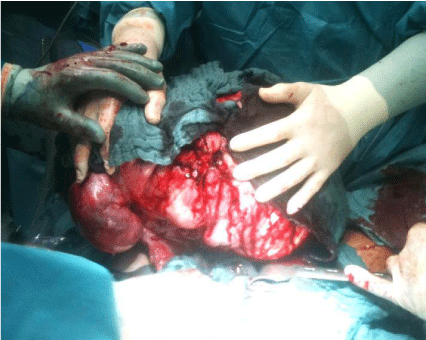
Figure 7: Removal of tumor.
Source: Patient´s clinical chart.
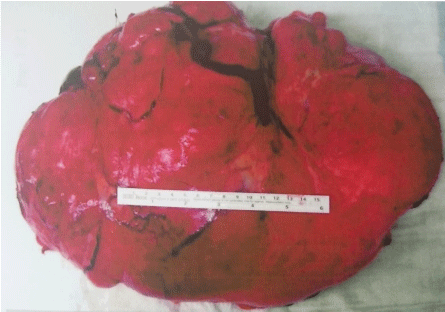
Figure 8: Tumor pathology.
Source: Patient´s clinical chart.
As the patient wished to preserve her fertility, only adhesiolysis, total tumor resection with further reconstruction of the uterine left wall, multiple cystectomies of peritoneal lesions and correction of endometriotic foci were performed. Intraoperative transfusions were necessary (6 U RG, 4U FFP). There were no postoperative complications.
At pathological analysis, the tumor weighed 8380g and had a soft consistency. The cut surfaces were pale-gray alternating with tan to yellow areas and showed some necrosis-like foci, with occasional whorly aspect to the tissue. Residual uterine tissue was not detectable. Cytologically, ascites fluid contained mesothelial cells with round- to oval-shaped and slightly eccentrically-lying nuclei. Tissue samples showed a myxoid mesenchymal tumor with alternating cellularity (Figure 9), plump-expansive and often permeable growth pattern, focalangioinvasion, with generally low but focally higher mitotic activity (Figure 10) and individual atypical mitoses. Nuclei had even contours and a rather fine chromatin pattern. There were small areas of hemorrhage, but true necrosis could not be found.
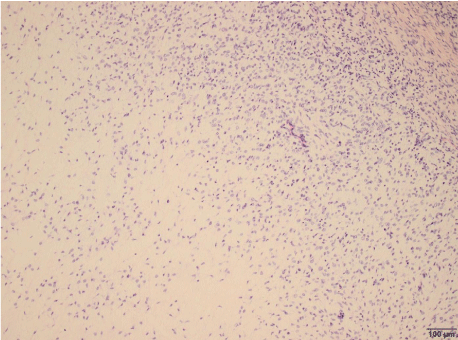
Figure 9: Endometrial stromal sarcoma with sparse cellularity in myxoid
areas (lower left).
Original magnification x100.
Source: Patient´s clinical chart .
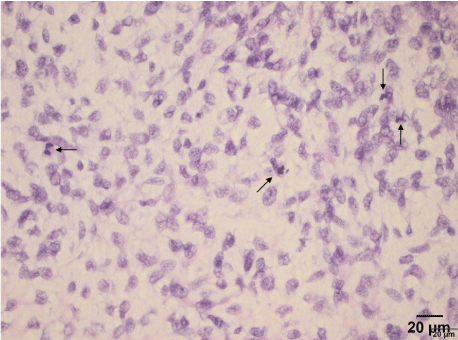
Figure 10: More cellular and frankly atypical area. Four mitotic figures can
be seen (arrows).
Original magnification x400.
Source: Patient´s clinical chart.
By immunohistochemistry, the tumor showed weak CD10 and strong Cyclin D1expression (Figure 11), with more than 70 % of nuclei labelled by the latter marker. Antibody Ki67 showed a growth fraction of 40 %. The tumor was negative for cytokeratins (AE1/ AE3), EMA, H-Caldesmon, Desmin, DOG-1, HMB45, Melan-A, S100, CD32, CD34, CD99, CD117, WT1, as well as for estrogen and progesterone receptors. In conclusion and despite the absence of steroid receptor expression, a diagnosis of stromal sarcoma, myxoid variant, was favored. While low-grade variants of myxoid endometrial stromal sarcoma are well known, the massive expression of cyclin D1 pointed towards a rare case of High-Grade Endometrial Stromal Sarcoma (HGESS) of myxoid variant.
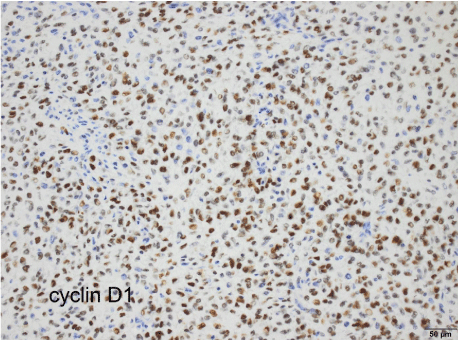
Figure 11: ajwh-v2-id1012-g011
For this reason, genetic testing was performed by Dr. E. Oliva and Dr. Sheng Xiao from Massachusetts General Hospital, confirming an unbalanced YWHAE rearrangement with loss of the 3’ region, as detected by fluorescence in situ hybridization (FISH) in 86 % of nuclei. FISH evaluation for YWHAE rearrangement was performed on 5 micron tissue sections with homebrew probes specific for the 5’ and 3’ regions of YWHAE, and mapping17p13.3, a characteristic 10,17translocation. These YWHAE t (10;17) (q22;p13.3) rearrangements, as well as the massive Cyclin D1 expression, are typical to HGESS profile (1). As a result of these findings, in combination with the relatively blandmorphology, a rare case of HGESS of myxoid variant, FIGO Stage II (T2, N0, M0) was diagnosed. Subsequently, a total hysterectomy was recommended, but was refused by the patient because of family planning, with the agreement to have adequate, regular follow-up.
Discussion
HGESS are rapidly growing neoplasms, most cases exhibiting extrauterine disease at time of diagnosis. Invasion of myometrial veins is common, combined in some cases with extension through ovarian veins into the inferior vena cava, occasionally producing a cavaltumoral thrombus, intracavitary cardiac extension, or invasion to the abdominal aorta. Other sites of spread are peritoneum and lungs [7]. The tumor in this case was associated with endometriosis, yet remarkably, despite its exceptionally large size, no invasion of surrounding structures was found.
No specific presurgical imaging or clinical criteria exist that help differentiate a benign tumor from a sarcoma [4]. In our case, there was no sign of malignancy detected on ultrasound or CT before surgery. On ultrasound imaging, sarcomas appear as large solid masses, frequently described as myomas.With Magnetic Resonance Imaging (MRI) sarcomas are described as large, heterogeneous masses having irregular contours, sometimes with degenerative changes, seen as delineated lesions of low signal intensity on T1 and T2-weighted images [2]. As malignancy cannot be ruled out before operation, procedures such as morcellation imply potential risk of tumor cell spillage, prompting the rationale for recommending use of endo bags during such procedures [4,8].
Therefore, diagnosis of ESS is made only after histopathological analysis of tumor tissue. Histologically, HGESS retain evidence of endometrial stromal derivation but display high-grade round-cell morphology [1]. At immunohistochemical analysis, they typically present with high Cyclin D1, low CD10 expression. At genomic analysis, HGESS harbor the YWHAE-FAM22 genetic fusion as a result of the translocation t(10;17) (q22;p13) [9-11]. The tumor in this case had relatively bland morphology, high Cyclin D1 expression and the characteristic YWHAE t(10;17) (q22;p13.3) rearrangements, leading to the diagnosis of HGESS of myxoid variant.
The standard treatment of primary and recurrent HGESS is surgery [12-16]. En-bloc total hysterectomy with bilateral salpingooophorectomy is recommended for young and postmenopausal women. Pelvic lymphadenectomy is recommended in selected cases where disease is found outside the uterus, but not routinely indicated in patients with small tumors with no macroscopic involvement, because no difference exists in patient survival rates. Fertility preserving surgery is not advised, but could be chosen by a well-informed patient, as was the decision of our patient after being informed of her final diagnosis.
Hormonal replacement therapy after oophorectomy is not recommended. Radiotherapy, chemotherapy or tyrosine kinase inhibitors as adjuvant therapy for primary, metastatic or recurrent disease show limited survival and relapse-free survival rates, but could be considered in certain cases [9,12,13].
According to the information provided by the patient, who lives abroad, she developed a new tumor in the abdominal cavity six months later, which was resected in a new fertility preserving surgery. She was not able to conceive within this time.
Conclusion
The present case of HGESS as a myxoid variant is unique for its several noteworthy features. This case highlights the limitations of presurgical imaging techniques, and the importance of immunohistochemistry and genetic testing for accurate diagnosis of EST, as without these, diagnosis of HGESS myxoid variant could not have been made. Accurate diagnosis is critical for determining patient-specific therapy and prognosis, and also for counselling patients who decide to conserve their fertility despite facing a highgrade tumor.
References
- Kurman RJ. International Agency for Research on Cancer, World Health Organization. WHO classification of tumours of female reproductive organs. 4th edn. Lyon: International Agency for Research on Cancer. 2014.
- Tavassoli FA, Devilee P. WHO Classification of Tumours: pathology and genetics of tumours of the breast and female genital organs. Lyon: International Agency for Research on Cancer. 2003.
- The ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014; 25:102-112.
- Beckmann MW, Juhasz-Böss I, Denschlag D, Gaß P, Dimpfl T, Harter P, et al. Surgical Methods for the Treatment of Uterine Fibroids - Risk of Uterine Sarcoma and Problems of Morcellation: Position Paper of the DGGG. Geburtshilfe Frauenheilkd. 2015; 75: 148-164.
- Ducimetière F, Lurkin A, RanchÈre-Vince D, Decouvelaere AV, PÈoc’h M, Istier L, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011. 6: e20294.
- Lee CH, Mariño-Enriquez A, Ou W, Zhu M, Ali RH, Chiang S, Amant F. The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol. 2012; 36: 641-653.
- Busuito BC, West CA, Rasool N, Rogers C. Endometrial stromal sarcoma invading the abdominal aorta treated with aortic replacement. J Vasc Surg. 2012; 55: 844-846.
- U.S. Food and Drug Administration. Updated laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. 2014.
- Thway K, Fisher C. Histopathological diagnostic discrepancies in soft tissue tumours referred to a specialist centre. 2009.
- Sardinha R, Hernández T, Fraile S, Tresserra F, Vidal A, Gómez MC, et al. Endometrial stromal tumors – immunohistochemical and molecular analysis of potential targets of tyrosine kinase inhibitors. Clinical Sarcoma Research. 2013; 3: 3.
- Lee CH, Hoang LN, Yip S, Reyes C, Marino-Enriquez A, Eilers G, et al. Frequent expression of KIT in endometrial stromal sarcoma with YWHAE genetic rearrangement. Mod Pathol. 2014; 27: 751-757.
- National Cancer Institute. Uterine Sarcoma Treatment. 2015.
- El-Khalfaoui K, du Bois A, Heitz F, Kurzeder C, Sehouli J, Harter P. Current and future options in the management and treatment of uterine sarcoma. Ther Adv Med Oncol. 2014; 6: 21-28.
- Philip CA, Pautier P, Duffaud F, Ray-Coquard I. High-grade undifferentiated sarcomas of the uterus: diagnosis, outcomes, and new treatment approaches. Curr Oncol Rep. 2014; 16: 405.
- Dizon DS, Birrer MJ. Advances in the diagnosis and treatment of uterine sarcomas. Discov Med. 2014; 17: 339-345.
- Grimer R, Judson I, Peake D, Seddon B. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010; 2010: 506182.