
Research Article
Austin J Womens Health. 2017; 4(1): 1025.
Iron Status in Relation to Oral Contraceptive Use in Women of Reproductive Age
Gellert S* and Hahn A
Institute of Food Science and Human Nutrition, Leibniz University Hannover, Germany
*Corresponding author: Gellert S, Leibniz University of Hannover, Institute of Food Science and Human Nutrition, Am Kleinen Felde 30, 30167 Hannover, Germany
Received: July 07, 2017; Accepted: August 11, 2017; Published: August 18, 2017
Abstract
This study investigated the iron status in women of reproductive age in relation to Oral Contraceptive (OC) use. 178 women (18-34 years) who had never been pregnant and did not take iron supplements were sampled as part of a cross-sectional study to determine nutrient status in different life stages (DRKS00004789). Iron status was assessed by haemoglobin, ferritin, soluble transferrin receptor (sTfR) and sTfR-ferritin index. Frequency of anaemia (haemoglobin < 12 g/dL) was 2.3 % and that of depleted iron stores without anaemia (ferritin < 20 µg/L) was 14.1 %. In multiple linear regression models, OC use was associated with higher ferritin concentration (B = 0.144, p = 0.037), especially the fourth progestin generation (B = 0.177, p = 0.015). Further determinants of lower ferritin concentration were higher intensity of menstruation (B = -0.201, p = 0.001), lower time since last period (B = -0.004, p = 0.024), blood donation (B = -0.360, p = 0.003) and vegetarian diet (B = -0.206, p = 0.042). Although the prevalence of anaemia was low, women of reproductive age should ensure adequate intake of highly available iron because depleted iron stores increase the risk of anaemia.
Keywords: Iron; Ferritin; Haemoglobin; Soluble Transferrin Receptor; Oral Contraceptive; Women of Reproductive Age
Abbreviations
AE: Non-Iron-Deficiency Anaemia; BMI: Body Mass Index; ID: Iron-Deficient non-anaemia; IDA: Iron-Deficiency Anaemia; IDE: Iron-Deficient Erythropoiesis; OC: Oral Contraceptives; SD: Standard Deviation; sTfR: Soluble Transferrin Receptor; sTfR-F index: Soluble Transferrin Receptor Ferritin Index; VitaMin Femin: Vitamin and Mineral Status among German Women
Introduction
Worldwide, the risk of anaemia (haemoglobin < 12 g/dL) is estimated at 29 % (496.3 million) in non-pregnant women aged 18 to 49 years [1]. Anaemia is caused by inadequate iron balance, which initially results in reduced iron stores (depressed ferritin levels) [2]. An iron deficiency without anaemia may already result in impaired neurocognitive functions [3]. Progressive iron deficiency leads to restricted erythropoiesis (increased soluble transferrin receptor [sTfR]). Further imbalance may lead in a deficiency of haemoglobin (anaemia) [2].
Iron status is particularly important among women of reproductive age who want to become pregnant because of the increased iron requirements during pregnancy [4] and the maternal and fetal health [5]. However, women of reproductive age are considered at-risk for iron deficiency [6] as menstruation-related blood losses strongly impact iron status [7]. The use of Oral Contraceptives (OCs) is associated with shorter bleeding times [8] and less blood loss [9] and may therefore positively affect iron status, as has been observed in a few studies [8,10] but the data situation is inconsistent [11].
Iron status also depends on the availability of iron from food. Both the chemical form [12] and the presence of inhibiting and enhancing factors influence the iron absorption [13]. Moreover, iron absorption from food depends on iron status [13] and Body Mass Index (BMI) [14]. Blood donations [15] and endurance sports participation [16] also affect the iron status. As a result, the assessment of iron status via iron-specific laboratory test (e.g., haemoglobin, ferritin, soluble transferrin receptor and soluble transferrin-ferritin index) is more appropriate than the evaluation of iron intake.
The present study describes the iron status in women of reproductive age in relation to OC use and in consideration of other factors that might influence the iron status.
Material and Methods
Subjects and study design
Subjects were recruited as part of the nationwide, cross-sectional, multicentre VitaMin Femin study (vitamin and mineral status among German women), which determined the status of selected nutrients in women at different life stages (n = 2367). The cross-sectional study was conducted in cooperation with 125 study sites (general practitioners and gynaecologists) between April 2013 and March 2015. Study design and implementation were conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. The study protocol was approved by the ethics commission of the Medical Chamber of Lower Saxony (26.03.2013) and every involved ethic commission from the different study sites. The study was registered in the German Clinical Trial Register with the identification number DRKS00004789 [17].
Iron status was measured in a subgroup of 192 women of reproductive age who had never been pregnant and did not take iron supplements. Of these, one subject was excluded from the analysis due to missing data on the type of OC used. Another 13 subjects with intrauterine devices or vaginal ring use were excluded, as the study aimed to compare the influence of OC use to non-use. Therefore, the study population included 178 women.
Iron indices
Iron status was assessed by haemoglobin, serum ferritin, sTfR and sTfR-ferritin (sTfR-F) index. The haemoglobin determination was conducted using sodium lauryl sulphate, a photometric method. Ferritin reflects iron stores [18] and was measured in serum by electrochemiluminescence immunoassay (cobas®, Roche Diagnostics, Mannheim, Germany). Ferritin is an acute phase protein and can be increased by inflammation [18], therefore sTfR – an indicator of tissue iron deficiency (Skine et al. 1990) – was also assayed in serum by immunonephelometry (BN II/BN ProcSpec® System, Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany). STfR was used to calculate the sTfR-F index (sTfR/log10 ferritin), which reflects total body iron [20].
Iron status was categorized by the cut-offs for anaemia (haemoglobin < 12 g/dL) and depleted or absent iron stores (ferritin < 15 µg/L) [21]. Moreover, as physical performance is already decreased at ferritin levels < 20 µg/L [22], this cutoff was used for reduced iron stores. Iron erythropoiesis is restricted at increased sTfR-F index [20]. As the measurement of sTfR is highly method dependent, the laboratory-dependent value for sTfR-F index was used (> 1.54) (N Latex sTfR, 2011, Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany). A severe risk of iron overload exists at a ferritin level > 150 µg/L [21].
Therefore, the following classifications were used: Iron-Deficient non-anaemia (ID) (ferritin < 15 µg/L, haemoglobin ≥ 12 mg/L, sTfR-F index ≤ 1.54), Iron-Deficient Erythropoiesis (IDE) (ferritin < 15 µg/L, haemoglobin ≥ 12 g/dL, sTfR-F index > 1.54), Iron-Deficiency Anaemia (IDA) (ferritin < 15 µg/L, haemoglobin < 12 g/dL, sTfR-F index > 1.54) and non-iron-deficiency Anaemia (AE) (ferritin > 15 µg/L, haemoglobin < 12 g/dL, sTfR-F index ≤ 1.54).
Factors influencing iron status
Different data were collected using a questionnaire (self-report) to consider factors that might influence the iron status: (1) OC use [8,10] (non-OC users vs. OC users), (2) OCs were classified by ethinyl estradiol concentration and progestin generation (second: levonorgestrel and norgestimate; third: desogestrel; new progestine with antiandrogen activity were classified as fourth: drospirenone, nomegestrol acetate, dienogest [23], chlormadinone acetate and cyproterone acetate [24]), (3) blood donation [15], (4) dietary pattern [13] (omnivore vs. non-omnivore [vegetarian and vegan]), (5) menstruation [25] (average duration in days, intensity [amenorrhoea, less-intense, more-intense, strong-intense] and time since last menstruation period), (6) smoking habits [26] and (7) BMI (calculated by weight and height) [14]. The group of blood donation included subjects which donated blood in the last twelve month before their involvement in the study. BMI was divided according to the WHO classification [27]. Women were classified as having amenorrhea if the last menstruation has been more than 60 days ago or they had long cycles.
Total study population (n = 178)
Non OC-users (n = 58)
OC-users (n = 120)
P value
Age (years)
Mean ± SD
23.0 ± 3.5
23.7 ± 3.9
22.7 ± 3.3
0.177a
BMI (kg/m²)
Mean ± SD
22.6 ± 3.8
22.8 ± 4.5
22.6 ± 3.4
0.727a
Intensity of menstruationc
N (%)
20 (11.4)
4 (7.0)
16 (13.4)
0.024b
Amenorrhoea
32 (18.2)
5 (8.8)
27 (22.7)
Less-intense
109 (61.9)
40 (70.2)
69 (58.0)
Normal-intense
15 (8.4)
8 (14.0)
7 (5.9)
More-intense
Last menstruation (days)d
Mean ± SD
15.7 ± 9.1
14.9 ± 8.6
16.2 ± 9.5
0.160a
Duration of menstruation (days)d
Mean ± SD
4.9 ± 1.2
5.1 ± 1.2
4.8 ± 1.1
0.558a
Donate bloode
N (%)
13 (7.3)
2 (3.4)
11 (9.2)
0.165b
Food patterne
Omnivore
N (%)
156 (87.6)
47 (81.0)
109 (91.6)
0.041b
Non-omnivoref
21 (11.8)
11 (19.0)
10 (8.4)
Smoking
N (%)
44 (24.7)
12 (20.7)
32 (26.7)
0.386b
Abbreviations: BMI, body mass index; OC, oral contraceptive
a Mann Whitney U test
b Chi square test
c n = 176
d n = 156 due to subjects with amenorrhoea (n = 20) and missing data (n = 2)
e n = 177
f three vegans
Table 1: Description of study population.
Statistical analysis
The statistical analyses were performed in the Statistical Package for the Social Sciences (SPSS) software version 22.0 (SPSS, Inc., Chicago, Illinois, USA). The results are presented as the mean ± standard deviation (± SD), range (minimum - maximum), frequency and percentage. The Kolmogorov-Smirnov test was used to analyse the normal distribution. Significant differences between subgroups were tested by Kruskal Wallis tests (> two groups) or nonparametric Mann Whitney U tests (two groups) for abnormally distributed data and by univariate ANOVA (> two groups) or t-tests for independent subsamples (two groups) for normally distributed data. To evaluate differences between categorical variables, Chi square tests were performed. Due to the skewed distribution of ferritin, a square root transformation was used for the multiple linear regression analysis. The models can be regarded as robust, as the forward and backward selection showed equal variable combinations. The significance level was p < 0.05.
Results
Subject characteristics
The characteristics of the study population are shown in Table 1. Here, 67.4 % (n = 120) of the study population used OCs. Frequencies of amenorrhea and low-intensity menstruation were significantly higher in OC users than in non-users (p = 0.024). Omnivorous dietary pattern were more common in OC users (p = 0.041). Among OC users, five subjects used progestin-only pills. Regarding the progestin generation, 24.2 % (n = 29) took second-generation OCs, 18.3 % (n = 22) third-generation OCs and 57.5 % (n = 69) fourth-generation OCs.
The majority of the total study population were normal weight (BMI 18.5 - 24.9 kg/m2, 71.9 %, n = 128) and only 5.6 % (n = 10) had obesity (BMI ≥ 30 kg/m2), which distribution did not vary between OC users and non-users (p = 0.158).
N
Haemoglobin (g/dL)
P value
Ferritin (µg/L)
P value
STfR (mg/L)
P value
STfR-F index
P value
Total
178
Mean ± SD
13.7 ± 0.9
/
51.5 ± 35.9
/
1.18 ± 0.30
/
0.78 ± 0.41
/
OC use
0.417a
0.029b
0.548b
0.156b
Non-users
58
Mean ± SD
13.7 ± 0.9
41.8 ± 23.7
1.20 ± 0.32
0.82 ± 0.42
Users
120
Mean ± SD
13.6 ± 0.9
56.2 ± 39.7
1.16 ± 0.29
0.76 ± 0.41
Ethinylestradiol concentration in OC userse
0.02 mg
35
Mean ± SD
13.7 ± 0.7
0.417a
59.8 ± 53.3
0.662b
1.17 ± 0.39
0.923b
0.78 ± 0.55
0.724b
≥ 0.03 mg
80
Mean ± SD
13.6 ± 0.6
54.0 ± 32.2
0.75 ± 0.34
0.76 ± 0.41
Progestin generation in OC users
Second
29
Mean ± SD
13.8 ± 0.7
0.605b
58.8 ± 58.3
0.186c
1.13 ± 0.43
0.103c
0.79 ± 0.61
0.280c
Third
22
Mean ± SD
13.6 ± 0.9
47.9 ± 32.0
1.21 ± 0.26
0.84 ± 0.43
Fourth
69
Mean ± SD
13.6 ± 0.9
57.7 ± 31.9
1.16 ± 0.22
0.72 ± 0.21
Intensity of menstruationf
Amenorrhea
20
Mean ± SD
13.8 ± 0.9
0.359d
61.7 ± 32.8
0.005c
1.17 ± 0.18
0.066c
0.70 ± 0.16
0.020c
Less-intense
32
Mean ± SD
13.9 ± 1.0
63.6 ± 44.9
1.11 ± 0.19
0.67 ± 0.20
Normal-intense
109
Mean ± SD
13.6 ± 0.8
48.4 ± 33.6
1.17 ± 0.31
0.78 ± 0.39
More-intense
15
Mean ± SD
13.8 ± 1.1
34.0 ± 24.9
1.41 ± 0.48
1.16 ± 0.78
Donate bloodg
Non-donors
164
Mean ± SD
13.7 ± 0.9
0.402a
53.5 ± 36.2
0.003b
1.16 ± 0.26
0.063b
0.75 ± 0.32
0.004b
Donors
13
Mean ± SD
13.5 ± 1.0
28.8 ± 21.6
1.14 ± 0.57
1.23 ± 0.90
Food patterng
Omnivore
156
Mean ± SD
13.7 ± 0.9
0.862d
53.6 ± 37.1
0.048c
1.17 ± 0.31
0.206c
0.78 ± 0.43
0.034c
Non-omnivore
21
Mean ± SD
13.6 ± 0.7
37.5 ± 21.4
1.21 ± 0.21
0.83 ± 0.23
Smoking
Non-smoker
134
Mean ± SD
13.6 ± 0.9
0.292a
49.1 ± 32.4
0.315b
1.21 ± 0.32
0.019b
0.82 ± 0.45
0.046b
Smoker
44
Mean ± SD
13.8 ± 0.8
58.7 ± 44.6
1.09 ± 0.22
0.68 ± 0.22
Abbreviations: OC, oral contraceptive; STfR, soluble transferrin receptor; STfR-F index, soluble transferrin receptor ferritin index
a t-Test for independent subsamples
b Mann Whitney U test
c Kruskal Wallis test
d Univariate ANOVA
e n = 115 due to five used progestin-only pills
f n = 176
g n = 177
Table 2: Haemoglobin, ferritin, sTfR-concentration and sTfR-F index in total study group and depending on determinants of iron status.
Iron status
In the total study population, the average haemoglobin concentration was 13.7 ± 0.9 g/dL, ferritin concentration 51.5 ± 35.9 µg/L, sTfR concentration 1.18 ± 0.30 mg/L and sTfR-F index 0.78 ± 0.41 (Table 2). With regard to OCs, only ferritin concentration was affected by the use of OCs, resulting in significantly higher concentrations in users than in non-users (p = 0.029) (Table 2). Ferritin levels did not vary across ethanol estradiol concentrations among OC users. Interestingly, ferritin values were only significantly higher among users of fourth-generation compared to non-users (p = 0.004). Among OC users, iron status did not differ by estradiol concentration or progestin generation.
Most of the iron markers did not depend on possible influencing factors in the total study population (intensity of menstruation, blood donation, dietary pattern, and tobacco use (Table 2), BMI classification [data not shown]). However, significantly lower ferritin concentrations and higher sTfR-F index values were seen among women with intense menstruation (ferritin: p = 0.005, sTfR-F index: p = 0.020), blood donors (ferritin: p = 0.003, sTfR-F index: p = 0.004) and non-omnivores (ferritin: p = 0.048, sTfR-F index: p = 0.034). The sTfR concentration and sTfR-F index value were significantly lower in smokers than in non-smokers (sTfR: p = 0.019, sTfR-F index: p = 0.046).
Prevalence of cut-off values for iron status
Of the study population, 82.5 % had normal iron status. Iron deficiency without anaemia was present in 11.8 % (n = 21) of women and erythropoiesis was restricted in 2.3 % (n = 4) (IDE). Anaemia was present in 2.3 % (n = 4) of women, but only 0.6 % (n = 1) also had iron deficiency (IDA). The other 1.7 % (n = 3) showed anaemia without iron deficiency (AE). Another 1.1 % (n = 2) of women had a severe risk of iron overload.
The frequency of reduced iron stores (ferritin < 20 µg/L) was higher in women with more-intense menstruation than in women with less-intense menstruation (p = 0.004) and in blood donors (p = 0.007) (Figure 1). However, the prevalence did not vary across OC use (p = 0.252) or dietary patterns (p = 0.490, data not shown).
Regression coefficient
Standard- error
Standardized regression coefficient
P value
Constant
3.177
0.183
0.000
Non OC-users/OC users
0.144
0.069
0.154
0.037
Non OC-users/ second generation OC
0.073
0.053
0.157
0.169
Non OC-users/ third generation OC
0.120
0.087
0.142
0.172
Non OC-users/ fourth generation OC
0.177
0.072
0.220
0.015
Intensity of period (amenorrhea/less-/ normal-/more-intense)
-0.201
0.059
-0.361
0.001
Last period
-0.004
0.002
-0.232
0.024
Blood donation (no/yes)
-0.360
0.118
-0.218
0.003
Food pattern (omnivore/vegetarian)
-0.206
0.100
-0.144
0.042
OC, oral contraceptive.
a multiple linear regressions considering the terms OC use, intensity of period, last period, blood donation and food pattern
b n = 175, without vegans (n = 3)
Table 3: Determinants of square root ferritin concentration by multiple linear regressionsa,b.
Determinants of ferritin concentration
In the multiple linear regression analysis, OC use was positively associated with ferritin concentration (p = 0.037) (Table 3). However, a relationship was only seen for OCs of fourth-generation (p = 0.015). The menstruation intensity (p = 0.001), time since last menstruation (p = 0.024) and blood donation (p = 0.003) were negatively associated with ferritin levels (Table 3). With regard to dietary pattern, ferritin concentration was only associated with mixed and vegetarian diets (excluding vegan diets), with lower ferritin levels among vegetarians compared to omnivores (p = 0.042). Therefore, vegans were not included in the model.
Ethinyl estradiol concentration, average duration of menstruation, tobacco use and BMI did not contribute to the model and were therefore excluded from the multiple linear regressions.
Discussion
Our findings show that iron status was inadequate in 16.4 % of study subjects. Anaemia was prevalent in 2.3 % (haemoglobin < 12 g/dL), and 14.1 % had depleted iron stores without anaemia (ferritin < 20 µg/L). The prevalence of anaemia was not critical. However, with depleted iron stores, neurocognitive function can be reduced and the risk of anaemia increases [3].
Previous studies showed similar rates of iron deficiency (10 - 20 %) and anaemia (2 - 6 %); however, different cut-offs for iron deficiency were used (ferritin < 12 µg/L, < 15 µg/L or < 16 µg/L) [8,28,29]. We thus find a lower prevalence of depleted iron status than these trials.
OC use (p = 0.252a), intensity of period (p = 0.042a) and blood donation (p =
0.007a). OC, oral contraceptive; a Chi square test.
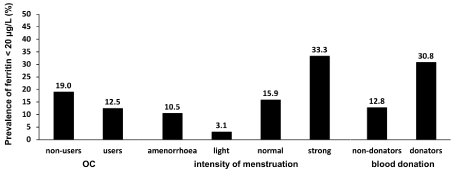
Figure 1: Frequency of reduced iron stores (ferritin < 20 μg/L) depending on
OC use (p = 0.252a), intensity of period (p = 0.042a) and blood donation (p =
0.007a). OC, oral contraceptive; a Chi square test.
OC use was only associated with higher ferritin concentrations and, therefore, with higher iron stores, whereas the prevalence of depleted iron stores (ferritin < 20 µg/L) was independent of OC use. Haile et al. also showed that risks of iron deficiency (sTfR > 43.9 nmol/L), iron deficiency anaemia (sTfR > 43.9 nmol/L and haemoglobin < 12 g/dL) and anaemia non-iron deficient (haemoglobin < 12 g/dL) are 49 %, 80 % and 58 % lower, respectively, in OC users than in non-users [10]. However, the prevalence of inadequate iron stores was higher in the study population of Haile et al. than in our population. Iron status may vary with the type of OC. Although oestrogen concentration had no effect in our study, interestingly, the progestin generation was associated with ferritin concentration. However, differences in ferritin concentration between non-users and OC users were only seen for the fourth-generation. In contrast to the other progestin generations, fourth-generation has antiandrogen activity [24], which results in a lower intermenstrual bleeding rate, lower intensity of menstrual bleeding and lower frequency of dysmenorrhoea [30]. This finding probably explains the non-existent influence of OC use on ferritin concentration, sTfR concentration and sTfR-F index found by Casabellata et al. Their study population used only third-generation OCs [11]. Therefore, OC use may result in smaller menstruation-related blood losses [8]. Iron losses are higher in women with menorrhagia than in women with normal menstruation (5.2 mg vs. 0.87 mg iron/cycle) [31], and 27 % and 60 % of women with strong menstrual bleeding show anaemia and iron deficiency, respectively [25]. Although details on menstruation were subjectively described in our study, the lower the intensity of menstruation and the longer the time since the last menstruation, the higher the observed ferritin concentration. Menstruation-related blood loss is an independent contributor to ferritin levels, as blood loss strongly depends mainly on individual factors [31] and also intra-individual [32].
Regarding diet, omnivores had significantly higher ferritin concentrations and lower sTfR-F index values than vegetarian, as shown by Leonard et al. [33]. These results conflict with the iron intake, which is surprisingly highest among vegans followed by vegetarians and lowest among omnivores [34]. However, iron absorption from a vegetarian diet is lower than from an omnivorous diet [35]. Furthermore, vegetarians consume higher amounts of iron inhibitors, such as phytic acid in soy protein [36] and dietary fiber [37].
Blood donation was associated with depleted iron stores and higher sTfR-F index values, which can result in an impaired erythropoiesis with increasing iron imbalance. Moreover, depleted iron stores increase the risk of iron deficiency erythropoiesis, as shown by Cable et al. – 62 % of the blood donors had iron deficiency erythropoiesis (sTfR ≥ 2.07) [15].
In obese women, iron absorption is restricted by obesity-related inflammation compared to normal-weight women with similar iron intake [14]. However, the ferritin concentration is higher in obese women [38]. We find no influence of BMI on ferritin status.
This study has some limitations. First, the prevalence of reduced iron stores may be higher than observed as serum ferritin concentration increase with infection and inflammation [19]. Therefore, we used the sTfR concentration, which is not affected by inflammation. Second, information about the intensity and duration of menstruation were subjective descriptions. Furthermore, although we have considered the type of diet, dietary records would be more appropriate. Moreover, sTfR concentration is influenced by physical activity [16]. However, this factor was not considered in this study.
Conclusion
In conclusion, of the various factors considered (menstruation, blood donation, food pattern), use of fourth-generation OC is positively correlated with ferritin concentration, which reflects higher iron status. Overall, anaemia was prevalent in 2.3 % and reduced iron stores in 14.1 % in women of reproductive age who did not take iron supplements. Menstruation-related blood losses, in particular, negatively affect iron status [7] and during pregnancy iron requirements are particularly necessary [5]. For those reasons women of reproductive age should ensure adequate intakes of highly available iron.
Acknowledgement
We would like to thank the physicians and participants who contributed their time to this study. The study was supported by Rottapharm Madaus GmbH (Cologne, Germany) – now a part of Meda AB (Bad Homburg, Germany). The authors are solely responsible for the design and conduct of the study, collection, management, analysis, and interpretation of the data, as well as preparation of the manuscript. All authors had full access to the data and take responsibility for its integrity. All authors have read and agree with the manuscript as written.
References
- WHO [World Health Organization]. The global prevalence of anaemia in 2011. Geneva, Switzerland. 2015.
- Clark SF. Iron deficiency anemia. Nutr Clin Pract. 2008; 23: 128-141.
- Coad J, Conlon C. Iron deficiency in women: assessment, causes and consequences. Curr Opin Clin Nutr Metab Care. 2011; 14: 625-634.
- Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000; 72: 257S-264S.
- Breymann C. Iron deficiency anemia in pregnancy. Semin Hematol. 2015; 52: 339-347.
- Fonseca C, Marques F, Robalo NA, Belo A, Brilhante D, Cortez J. Prevalence of anaemia and iron deficiency in Portugal: the EMPIRE study. Intern Med J. 2016; 46: 470-478.
- Harvey LJ, Armah CN, Dainty JR, Foxall RJ, Lewis DJ, Langford NJ, et al. Impact of menstrual blood loss and diet on iron deficiency among women in the UK. BJN. 2005; 94: 557.
- Milman N, Clausen J, Byg KE. Iron status in 268 Danish women aged 18-30 years: influence of menstruation, contraceptive method, and iron supplementation. Ann Hematol. 1998; 77: 13-19.
- Fraser IS, Jensen J, Schaefers M, Mellinger U, Parke S, Serrani M. Normalization of blood loss in women with heavy menstrual bleeding treated with an oral contraceptive containing estradiol valerate/dienogest. Contraception. 2012; 86: 96-101.
- Haile ZT, Teweldeberhan AK, Chertok IRA. Association between oral contraceptive use and markers of iron deficiency in a cross-sectional study of Tanzanian women. Int J Gynaecol Obstet. 2016; 132: 50-54.
- Casabellata G, Di Santolo M, Banfi G, Stel G, Gonano F, Cauci S. Evaluation of iron deficiency in young women in relation to oral contraceptive use. Contraception. 2007; 76: 200-207.
- Hallberg L, Bjorn-Rasmussen E, Howard L, Rossander L. Dietary heme iron absorption. A discussion of possible mechanisms for the absorption-promoting effect of meat and for the regulation of iron absorption. Scand J Gastroenterol. 1979; 14: 769-779.
- Collings R, Harvey LJ, Hooper L, Hurst R, Brown TJ, Ansett J, et al. The absorption of iron from whole diets: a systematic review. Am J Clin Nutr. 2013; 98: 65-81.
- Cepeda-Lopez AC, Osendarp SJ, Melse-Boonstra A, Aeberli I, Gonzalez-Salazar F, Feskens E, et al. Sharply higher rates of iron deficiency in obese Mexican women and children are predicted by obesity-related inflammation rather than by differences in dietary iron intake. Am J Clin Nutr. 2011; 93: 975-983.
- Cable RG, Glynn SA, Kiss JE, Mast AE, Steele WR, Murphy EL, et al. Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2012; 52: 702-711.
- Di Santolo M, Stel G, Banfi G, Gonano F, Cauci S. Anemia and iron status in young fertile non-professional female athletes. Eur J Appl Physiol. 2008; 102: 703-709.
- Gellert S, Schuchardt JP, Hahn A. Higher omega-3 index and DHA status in pregnant women compared to lactating women - Results from a German nation-wide cross-sectional study. Prostglandins Leukot Essent Fatty Acids. 2016; 109: 22-28.
- Skikne BS, Flowers CH, Cook JD. Serum transferrin receptor: a quantitative measure of tissue iron deficiency. Blood. 1990; 75: 1870-1876.
- Di Thurnham, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr. 2010; 92: 546-555.
- Punnonen K, Irjala K, Rajamäki A. Serum Transferrin Receptor and Its Ratio to Serum Ferritin in the Diagnosis of Iron Deficiency. Blood. 1997; 89: 1052-1057.
- World Health Organization. Iron deficiency anaemia: assessment, prevention, and control. A guide for programme managers. Geneva, Switzerland. 2001.
- Brutsaert TD, Hernandez-Cordero S, Rivera J, Viola T, Hughes G, Haas JD. Iron supplementation improves progressive fatigue resistance during dynamic knee extensor exercise in iron-depleted, nonanemic women. Am J Clin Nutr. 2003; 77: 441-448.
- Golobof A, Kiley J. The current status of oral contraceptives: progress and recent innovations. Semin Reprod Med. 2016; 34: 145-151.
- Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, et al. Classification and pharmacology of progestins. Maturitas. 2003; 46: 7-16.
- Peuranpää P, Heliövaara-Peippo S, Fraser I, Paavonen J, Hurskainen R. Effects of anemia and iron deficiency on quality of life in women with heavy menstrual bleeding. Acta Obstet Gynecol Scand. 2014; 93: 654-660.
- Pynaert I, Bacquer D de, Matthys C, Delanghe J, Temmerman M, Backer G, et al. Determinants of ferritin and soluble transferrin receptors as iron status parameters in young adult women. Public Health Nutr. 2009; 12: 1775.
- WHO [World Health Organization]. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Commitee. Geneva, Switzerland: World Health Organization. 1995. (WHO Technical Report Series 854).
- Lahti-Koski M, Valsta LM, Alfthan G, Tapanainen H, Aro A. Iron status of adults in the capital area of Finland. Eur J Clin Nutr. 2003; 42: 287-292.
- Pynaert I, Delanghe J, Temmerman M, Henauw S de. Iron Intake in Relation to Diet and Iron Status of Young Adult Women. Ann Nutr Metab. 2007; 51: 172-181.
- Golbs S, Domhardt R, Radowicky S, Kaluzny Z, Wisser KH, T Zimmermann. Clinical findings with the oral contraceptive combination ethinylestradiol/dienogest in Poland. Methods Find Exp Clin Pharmacol. 2002; 24: 585-592.
- Napolitano M, Dolce A, Celenza G, Grandone E, Perilli MG, Siragusa S, et al. Iron-dependent erythropoiesis in women with excessive menstrual blood losses and women with normal menses. Ann Hematol. 2014; 93: 557-563.
- Astrup K, Olivarius NF, Moller S, Gottschau A, Karlslund W. Menstrual bleeding patterns in pre- and perimenopausal women: a population-based prospective diary study. Acta Obstet Gynecol Scand. 2004; 83: 197-202.
- Leonard AJ, Chalmers KA, Collins CE, Patterson AJ. The effect of nutrition knowledge and dietary iron intake on iron status in young women. Appetite. 2014; 81: 225-231.
- Clarys P, Deliens T, Huybrechts I, Deriemaeker P, Vanaelst B, Willem De Keyze, et al. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients. 2014; 6: 1318-1332.
- Hunt JR. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am J Clin Nutr. 2003; 78: 633-639.
- Weinborn V, Pizarro F, Olivares M, Brito A, Arredondo M, Flores S, et al. The Effect of Plant Proteins Derived from Cereals and Legumes on Heme Iron Absorption. Nutrients. 2015; 7: 8977-8986.
- Bach KM, Tetens I, Alstrup JAB, Dal TA, Milman N, Hels O, et al. A decrease in iron status in young healthy women after long-term daily consumption of the recommended intake of fibre-rich wheat bread. European Journal of Clinical Nutrition. 2005; 44: 334-340.
- Mujica-Coopman MF, Brito A, López de Romaña, Daniel, Pizarro F, Olivares M. Body mass index, iron absorption and iron status in childbearing age women. Journal of Trace Elements in Medicine and Biology. 2014; 30: 215-219.