
Original Article
Austin J Womens Health. 2019; 6(1): 1032.
Instant Analgesic Effect of Sublingual Progesterone Dilution in Fibromyalgia
Shilpa S¹* and Roby R²
¹Postdoctoral Studies, University of Mumbai, India
²Ex-Roby Institute, USA
*Corresponding author: Shilpa Shah, Postdoctoral Studies, University of Mumbai, Mumbai, India; E-mail: rescience_5@yahoo.co.in
Received: January 10, 2019; Accepted: February 25, 2019; Published: March 04, 2019
Abstract
Fibromyalgia is a disorder chiefly characterized by widespread musculoskeletal pain. It is more common in women than men. Its severity is linked to the menstrual cycle with worsening of symptoms prior to menses. Varied medications like pain relievers, antidepressants and anticonvulsants are in use to relieve this condition, but there is no recognized cure. With this background, findings of the present pilot study could provide new approach for pain relief for fibromyalgia sufferers and perhaps also for other pain disorders. Functional MRI-brain (fMRI-brain) was done to examine brain activity for pain relief in response to a single dose of 0.5mg sublingual progesterone dilution in a woman suffering from fibromyalgia. Her pain symptoms were of headache, severe eye pain, back pain, muscle and joint pain. On sublingual administration of 0.5mg progesterone dilution the patient reported complete pain relief and the fMRI-brain showed an area of prominent activation in the posterior cingulated gyrus. Progesterone being a neurosteroid can possibly act through ion channelassociated membrane receptors to elicit rapid changes in signaling that can occur within seconds resulting in instant resolution of physical pain.
Keywords: Progesterone; Analgesic; Pain; Fibromyalgia; Sublingual; fMRIbrain
Introduction
Fibromyalgia is estimated to affect 2-8% of the population [1]. Its pathology connects to dysfunction of muscles and connective tissue as well as functional abnormalities in the central nervous system. There is extensive research evidence to support the view that the central symptom of fibromyalgia, namely pain, result from processes in the central nervous system and the condition is referred to as a “central sensitization syndrome” [1-5]. The chronic pain of fibromyalgia is pervasive and persistent. As neither the etiology nor mechanism of fibromyalgia is currently well understood, it is a tough challenge for patients as well as physicians to manage the fibromyalgia related chronic pain till now [6,7].
Fibromyalgia affects women twice as often as men [1]. It is common among patients with premenstrual syndrome and shares similarities and features with premenstrual dysphoric syndrome [8,9]. Menstrual cycle related hormonal fluctuations could be affecting the expression and severity of symptoms of fibromyalgia. A recent study published in the Journal of pain [10] has reported that lower levels of testosterone and progesterone are significantly associated with increased pain in Fibromyalgia. Progesterone levels naturally fluctuate in relation to the menstrual cycle; rising during ovulation and falling again before menstruation. It is a neurosteroid that can be metabolized within all parts of the central nervous system [11]. It is a neuromodulator, with neuroprotective and neurogenic properties that can regulate neurotransmission and myelination [12]. The present study discovers instant analgesic effect of low dose sublingual progesterone dilution.
Methods
Study subject
The subject was a 54 years old female patient (date of birth 12 November 1953) suffering from widespread body aches since puberty at the age of 13. At the age of 30 her complaints were diagnosed as fibromyalgia with severe headache, eye pain, back pain, muscle and joint pain. The pain had often been extreme and would worsen premenstrually. Her pain could only poorly be managed with Narcotics. Her right eye pain was so worse that she was suggested enucleation. Her sleep was affected and so did her day to day life. In 2006 she got started on the sublingual progesterone dilution therapy.
Personalized sublingual progesterone dose determination
Progesterone 50 mg/ml USP (Schein Laboratories, Florham, N.J.) suspended in sesame oil was used. It was diluted with normal saline to produce the progesterone dilutions 0.05 μg, 0.5 μg, 5 μg, 0.05 mg, 0.5 mg. To achieve an even suspension, the dilutions were vigorously shaken at each stage of the preparation and before use. 0.1 ml of these dilutions was administered sublingually to the patient beginning from 0.05 μg stepping up to 0.5 mg. Following administration of the sublingual progesterone dilution under the patient’s tongue, the patient was instructed to hold the medicine for 5 seconds before swallowing. After an additional 15 seconds, the patient was asked to rate her pain symptoms on the scale of zero to ten with ten being the most severe pain and zero indicating no pain at all [13].
Subject preparation
She was requested not to use her sublingual progesterone dilution dose for 72 hours prior to the scanning session.
fMRI-brain protocol
The fMRI investigation was done as an outpatient procedure. On arrival the patient was screened for safety in the magnetic environment. After getting positioned under the scanner, she was asked to rate her pain score on the scale of zero to ten (with ten being the most severe pain and zero indicating no pain at all). Her pain score was noted. Under the scanner when she was head stable a capsule containing 0.5 mg progesterone dilution was placed on her pursed lips. It is now that the imaging was started. Initial baseline BOLD imaging was performed and while continuing with the imaging: the patient was instructed to take in the capsule, crush and hold it sublingually for 5 seconds, and subsequently at 15 seconds she was asked to rate the pain score again. This single event continuous imaging ended in about 5 minutes post 0.5 mg sublingual progesterone dilution. The fMRI system was Magnetom Trio 3.0 Tesla (Siemens). Pulse sequence type was Echo-Planar imaging with parallel imaging parameter Generalized Autocalibrating Partial. Acquisition orientations were Axial, Sagittal, Coronal and Three-Dimensional.
Results
Based on the patient’s feedback during the personalized progesterone dilution dose determination; sublingual dose of 0.5 mg progesterone dilution was found to be most effective in alleviating her pain symptoms. When the fMRI-brain protocol was planned, by now the patient was on 0.5 mg sublingual progesterone dilution every 12 hourly for two years (2006-2008). Over this period, she had relief from pain: her sleep had improved and was able to do her daily activities without any limitations. The eye pain relief was so absolute that the suggested enucleation of the right eye was not required. Post withdrawal of sublingual progesterone 0.5mg for 72 hours (prior to the start of fMRI-brain imaging) she reported average pain symptom score of 8 on the scale of 0 to 10 (with ten being the most severe pain and zero indicating no pain at all). During the fMRI-brain imaging, after administration of her regular dose of sublingual progesterone dilution 0.5 mg, in 15 seconds she reported 0 pain symptom score (on the scale of 0 t0 10 with ten being the most severe pain and zero indicating no pain at all).
The fMRI-brain outcome highlighting significant change in the brain region post sublingual progesterone dilution 0,5 mg and forthwith pain relief are illustrated in the underneath figures (Figure 1, Figure 2, Figure 3 and Figure 4). It will be supportive to know the chief finding prior to taking into consideration details of each of these figures. Chiefly, although scattered noise was identified the bold imaging following sublingual administration of progesterone dilution 0.5 mg showed more prominent activation in a single area measuring about 12 mm in the posterior cingulated gyrus nearly along the posterior margin of Brodmann’s area 31 and 24. Figure 1: Images 1, 2, 3, 4 are the initial baseline BOLD images. It was 2.26 seconds later the sublingual administration of progesterone dilution 0.5 mg that the posterior cingulated gyrus showed prominent activation which lasted for 6.21 seconds (Figure 1: Image 6 and Figure 4: Images 1 & 2). Figure 2: Image 2 shows administration of 0.5 mg sublingual progesterone dilution and simultaneous activation in the posterior cingulated gyrus. It was also endorsed in the three-dimensional view of the f-MRI brain images (Figure 3).
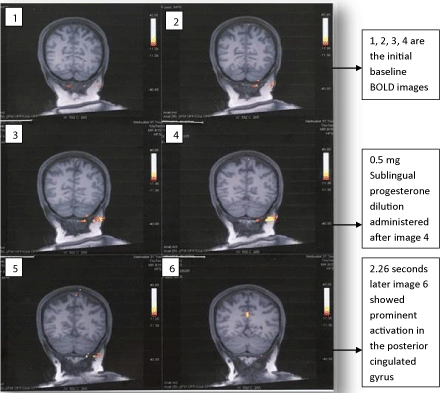
Figure 1: Dorsal Coronal f-MRI brain images of the fibromyalgia patient.
Images 1, 2, 3 and 4 display the baseline status.
Progesterone dilution 0.5 mg was sublingually administered after image 4.
2.26 seconds later the image 6 showed prominent activation in the posterior
cingulated gyrus. Image 5 and 6 are post-sublingual ladministration of the 0.5
mg progesterone dilution.
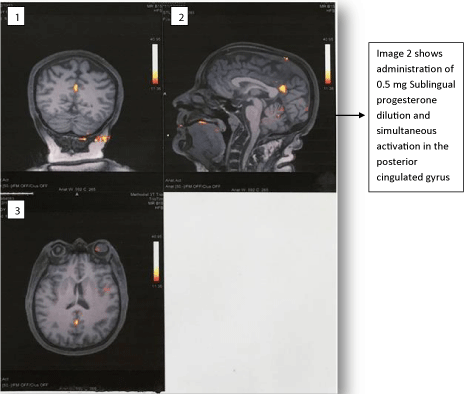
Figure 2: Different views of f-MRI brain images of the fibromyalgia patient
post sublingual administration of the 0.5 mg progesterone dilution. Image 1:
Dorsal Coronal view. Image 2: Sagittal view.
Image 3: Cranial Axial view.
All show prominent activation in the posterior cingulated gyrus.
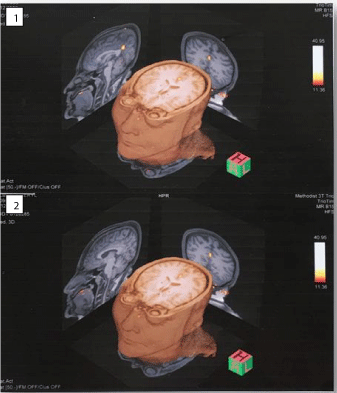
Figure 3: Three-Dimensional view of the f-MRI brain images of the
fibromyalgia patient post sublingual progesterone dilution 0.5 mg. Image 1
shows Sagittal view from the left with prominent activation in the posterior
cingulated gyrus. Image 2 shows Sagittal view from the right which does not
show any activated region. In both the images (1 & 2) the Coronal and Cranial
Axial views show prominent activation in the posterior cingulated gyrus.
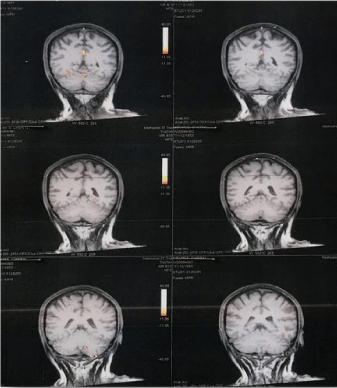
Figure 4: Ventral Coronal f-MRI brain images of the fibromyalgia patient
post sublingual progesterone dilution 0.5 mg. Image 1 and Image 2 shows
prominent activation in the posterior cingulated gyrus. Later images (3, 4, 5
and 6) do not show any activated region. Beginning from Image 6 in Figure
1 to Image 2 in the current figure the activation in posterior cingulated gyrus
lasted for 6.21 seconds.
Discussion
Diluted progesterone
The study subject had onset of fibromyalgia at puberty with premenstrual worsening of the pain symptoms. This indicated that premenstrual hormonal fluctuations may be the underpinning cause of her disease. Progesterone drop prior to the menstruation is linked to severity of symptoms in fibromyalgia [10]. Vincent K et al [14] from the university of Oxford, Uk have proposed a phenomenon called “luteal analgesia”. It advocates that the high estradiol high progesterone state indicative of ovulation would be associated with a reduction in the pain experience. Therefore, progesterone prescription could have assisted our subject for pain relief. But supplementation of progesterone to rectify the progesterone fall and hence pain is not a good idea. It could interfere with the natural process of menstruation plus there are other possible risks of hormone replacement therapy [15]. These limitations lead the authors to attempt a therapy with diluted progesterone via sublingual route [16].
Personalized sublingual progesterone dose determination
Progesterone effect is concentration as well as infusion mode dependent. Low pulsatile doses of the progeseteonr positively modulate the neurotansmission mechanisms, whereas in high doses it has negative effects [17]. In accordance of that, while practicing our designed personalized sublingual progesterone dose determination method we found that there was a particular dose, in the ascending order from 0.05 μg, 0.5 μg,, 5 μg, 0.05 mg, and 0.5 mg, to which the patient ranked highest relief on the pain scale i.e. either absolute or highest fall in the pain score. A dose higher than that was often found unsuitable: reversing the relief or worsening the pain. The results were motivating. The fibromyalgia patient’s pain symptoms were under control for 2 years with 0.5 mg sublingual progesterone dilution.
fMRI-brain
Consistent effect and efficacy of this low dose progesterone dilution in pain control; and its generalized analgesic effect on pain in different organs: headache, eye pain, back pain, muscle and joint pain, raised a research question: Is this therapy activating any area in brain leading to pain relief? To find an answer to this fMRI-brain was considered. fMRI is a powerful approach for probing the action of therapeutic drugs that act on the central nervous system [18] and is widely used to explore analgesic drugs as well as to study the molecular mechanisms of pain [19]. Event fMRI was pertinent for our study as it gives flexibility to design a protocol to estimate activation in response to a single event with specific question. As fMRI can find active areas of brain within a window of a few seconds its pace was in congruence with the rapidly acting sublingual progesterone dilution [20]. The study documents clinical usefulness of fMRI-brain to correlate objective treatment for subjective symptoms.
Analgesic effect of sublingual progesterone dilution
In 15 seconds of sublingual administration of 0.5 mg progesterone dilution, our study subject had reported absolute pain relief i.e. zero pain score from the average pain score of 8.0. Progesterone is a neurosteroid. At an appropriate determined dose it can possibly act through ion channel-associated membrane receptors to elicit rapid changes in signaling, that can occur within milliseconds to seconds, resulting in altered excitability of neurons and instant resolution of physical pain [21-23]. Hormones do influence brain in relation to pain. It has been reported that differences in pain perception are modulated by endogenous hormonal fluctuations [24,25]. An interesting longitudinal single subject fMRI-brain study published in the Frontiers in Neuroscience by Arlein K et al [26] has stated that progesterone mediates brain functional connectivity changes during the menstrual cycle. Tu et al [27,28] have reported that women suffering from severe menstrual pain have changes in brain regions associated with pain modulation, pain transmission, and affective experience generation.
Activation in the posterior cingulated gyrus
Post sublingual administration of the 0.5 mg progesterone dilution there was a single area of more prominent activation measuring about 12 mm in the posterior cingulated gyrus nearly along the posterior margin of Brodmann’s area 31 and 24 (Figures 1-4). This activation appeared 2.26 seconds after sublingual administration of the 0.5 mg progesterone dilution (Figure 1) and lasted for 6.21 seconds (Figures 1-4). German neuroscientist Dr Burkhart Bromm [29] has shown that the posterior cingulated cortex (which includes the entire cingulated gyrus) responds to nociceptive messages in about 220 milliseconds after the nociceptive stimulus is applied. This activity then move on to the medial and anterior portions of the cingulated cortex before subsiding in the frontal cortex which is about 300 milliseconds after the stimulus began. This speed of response to nociceptive stimuli by the posterior cingulate cortex draws parallel to our equally quick to occur study findings: pain relief within seconds and forthwith activation of the cingutated gyrus also within seconds. This indicates possibility of a bidirectional mechanism of pain awareness and dose reliant pain relief. This possibility is supported by a neuroimaging study in humans by Small et al [30], which concludes that “the only brain region that was active during both positive and negative compared with neutral conditions was the posterior cingulate cortex”. Additionally neuronal activity in posterior cingulate cortex is known to vary with pain, learning, reward, task engagement, internal processing of different cues with mindfulness, taste and episodic memory retrieval. [31-35]. While several different possibilities could relate to the prominent activation in posterior cingualte gyrus on fMRI-brain scan of our study subject, the analgesic effect of sublingual progesterone dilution on her fibromyalgia pain symptoms deserves more attention.
Conclusion
Forward inference of the present study is that in fibromyalgia patient the sublingual progesterone dilution has analgesic effect which catches on the fMRI-brain as activation in the posterior cingulated gyrus. It is important to note that as this novel finding is based on a pilot study, it necessitates replication with a larger sample size. Such a bigger study would require personalized sublingual progesterone dose decision for individual subject along with single event fMRI. The possible findings would be of immense benefit for patients suffering from fibromyalgia, and other menstrual cycle related hormone linked disorders. It could possibly open an entirely new way for pain management.
Special note: Second author of this study Dr Roby Russell passed away in September 2017.
Acknowledgment
Authors are thankful to the radiologist Dr DIX James E and the radiology technologist Krebs Martin L (Methodist Hospital, Department of Radiology San Antonio, Texas 78229) for the fMRIbrain investigation.
References
- Clauw DJ. Fibromyalgia. JAMA. 2014; 311: 1547-1555.
- Ngian GS, Guymer EK, Littlejohn GO. The use of opioids in fibromyalgia. Int J Rheum Dis. 2011; 14: 6-11.
- Häuser W, Eich W, Herrmann M, Nutzinger DO, Schiltenwolf M, Henningsen P. Fibromyalgia syndrome: classification, diagnosis, and treatment. Dtsch Arztebl Int. 2009; 106: 383-391.
- Clauw DJ, Arnold LM, McCarberg BH. The science of fibromyalgia. Mayo Clin Proc. 2011; 86: 907-911.
- Hawkins RA. Fibromyalgia: A Clinical Update. Journal of the American Osteopathic Association. 2013; 113: 680-689.
- Thomas MA. Pain management - the challenge. Ochsner J. 2003; 5: 15-21.
- Mao J. Challenges of managing chronic pain. BMJ. 2017; 356: j741.
- Amital D, Herskovitz C, Fostick L, Silberman A, Doron Y, Zohar J, et al. The premenstrual syndrome and fibromyalgia--similarities and common features. Clin Rev Allergy Immunol. 2010; 38: 107-115.
- Soyupek F, Aydogan C, Guney M, Kose SA. Premenstrual syndrome and fibromyalgia: the frequency of the coexistence and their effects on quality of life. Gynecol Endocrinol. 2017; 33: 577-582.
- Schertzinger M, Wesson-Sides K, Parkitny L, Younger J. Daily Fluctuations of Progesterone and Testosterone Are Associated With Fibromyalgia Pain Severity. J Pain. 2018; 19: 410-417.
- Schumacher M, Hussain R, Gago N, Oudinet JP, Mattern C, Ghoumari AM. Progesterone synthesis in the nervous system: implications for myelination and myelin repair. Front Neurosci. 2012; 6: 10.
- Schumacher M, Guennoun R, Robert F, Carelli C, Gago N, Ghoumari A, et al. Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination. Growth Hormone & IGF Research. 2004; 14: S18-S33.
- Kremer E, Atkinson JH, Ignelzi RJ. Measurement of pain: patient preference does not confound pain measurement. Pain. 1981; 10: 241-248.
- Vincent K, Stagg CJ, Warnaby CE, Moore J, Kennedy S, Tracey I. "Luteal Analgesia": Progesterone Dissociates Pain Intensity and Unpleasantness by Influencing Emotion Regulation Networks. Front Endocrinol (Lausanne). 2018; 9: 413.
- Regidor PA. Progesterone in Peri- and Postmenopause: A Review. Geburtshilfe Frauenheilkd. 2014; 74: 995-1002.
- Ruiz AD, Daniels KR. The effectiveness of sublingual and topical compounded bioidentical hormone replacement therapy in postmenopausal women: an observational cohort study. Int J Pharm Compd. 2014; 18: 70-77.
- Ramirez VD, Dluzen D. Is progesterone a pre-hormone in the CNS?. J Steroid Biochem. 1987; 27: 589-598.
- Shu-Feng Z. Functional magnetic resonance imaging is a powerful approach to probing the mechanism of action of therapeutic drugs that act on the central nervous system. Drug Des Devel Ther. 2015; 9: 3863-3865.
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013; 368: 1388-1397.
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One. 2010; 5: e15710.
- Frye CA. Neurosteroids’ effects and mechanisms for social, cognitive, emotional, and physical functions. Psychoneuroendocrinology. 2009; 34: S143-S161.
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992; 6: 2311-2322.
- Kim MJ, Shin HJ, Won KA, Yang KY, Ju JS, Park YY, et al. Progesterone produces antinociceptive and neuroprotective effects in rats with microinjected lysophosphatidic acid in the trigeminal nerve root. Mol Pain. 2012; 8: 16.
- Riley JL III, Robinson ME, Wise EA, Price DD. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999; 81: 225-235.
- Veldhuijzen DS, Keaser ML, Traub DS, Zhuo J, Gullapalli RP, Greenspan JD. The role of circulating sex hormones in menstrual cycle-dependent modulation of pain-related brain activation. Pain. 2013; 154: 548-559.
- Arélin K, Mueller K, Barth C, Rekkas PV, Kratzsch J, Burmann I, et al. Progesterone mediates brain functional connectivity changes during the menstrual cycle-a pilot resting state MRI study. Front Neurosci. 2015; 9: 44.
- Tu CH, Niddam DM, Chao HT, Chen LF, Chen YS, Wu YT, et al. Brain morphological changes associated with cyclic menstrual pain. Pain. 2010; 150: 462-468.
- Tu CH, Niddam DM, Yeh TC, Lirng JF, Cheng CM, Chou CC, et al. Menstrual pain is associated with rapid structural alterations in the brain. Pain. 2013; 154: 1718-1724.
- Burkhart Bromm. The involvement of the posterior cingulate gyrus in phasic pain processing of humans. Neuroscience Letters. 2004; 361: 245-249.
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001; 124: 1720-1733.
- Harris S, Jones M, Zheng Y, Berwick J. Does neural input or processing play a greater role in the magnitude of neuroimaging signals? Front Neuroenergetics. 2010; 2: 15.
- Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML. Posterior cingulate cortex: adapting behavior to a changing world. Trends Cogn Sci. 2011; 15: 143-151.
- Brewer JA, Garrison KA. The posterior cingulate cortex as a plausible mechanistic target of meditation: findings from neuroimaging. Ann N Y Acad Sci. 2014; 1307: 19-27.
- Szabó I, Hormay E, Csetényi B, Nagy B, Lénárd L, Karádi Z. Multiple functional attributes of glucose-monitoring neurons in the medial orbitofrontal (ventrolateral prefrontal) cortex. Neurosci Biobehav Rev. 2018; 85: 44-53.
- Cabeza R, Nyberg L. Neural bases of learning and memory: functional neuroimaging evidence. Curr Opin Neurol. 2000; 13: 415-421.