
Rapid Communication
Austin J Womens Health. 2019; 6(1): 1035.
Non-Surgical Management of Ectopic Pregnancies
Hakim H, Yaich R, Halouani S, Jouou S, Arfaoui R and Rachdi R*
Department of Gynecology and Obstetrics, Military Hospital of Tunis, Tunisia
*Corresponding author: Rachdi R, Head of Department of Gynecology and Obstetrics, Military Hospital of Tunis, Tunis, Tunisia
Received: September 05, 2019; Accepted: October 25, 2019; Published: November 01, 2019
Abstract
Ectopic pregnancy is a serious medical condition that can affect the patient’s fertility and even be life threatening in the most severe cases. The frequency of this condition is increasing on par with sexually transmitted diseases and medically assisted procreation techniques. This is a retrospective study concerning 91 cases of ectopic pregnancies among which 56 were treated medically (61.5% of cases) by 1mg of Methotrexate/Kg of body weight. The success rate was 62.5%. The best success predictive factor of medical treatment was HCG level below 1000mUI/ml. The average HCG negativation time was 21 days. 21 ectopic pregnancies didn’t respond to MTX (37.5%). The main fail criteria was the persistence of an HCG level above 15%, (52.3% of case). Fertility was preserved in 53% of cases during 5 years of observation.
Keywords: Ectopic pregnancy; Methotrexate; Non-Surgical; Sexually transmitted infections
Introduction
Ectopic Pregnancy (EP) is the implantation of the fertilized egg outside the uterine cavity. In normal pregnancies, the egg fertilization occurs in the ampulla of the fallopian tube. The fertilized egg then travels to the uterine cavity where the implantation occurs. During this journey, the implantation can occur outside the uterine cavity, frequently in the fallopian tube (mostly the ampulla) [1]. Ectopic pregnancy is a medical and surgical emergency; in the short term, it can be life threatening in case of tubal rupture [2]. However, it can have long-term complications as well, concerning mainly the patient’s fertility. Ectopic pregnancy is considered a public health problem, regardless of the country’s socioeconomic status. In recent years, the incidence of this situation has multiplied by 1.5 worldwide [3]. However, its morbidity and mortality have decreased. This can be explained by the development of diagnostic means, mainly HCG assay and transvaginal ultrasound. The treatment regimen was practically always surgical. Nevertheless, the therapeutic arsenal has known great changes. Ectopic pregnancies can now be treated medically, or simply monitored without any actual treatment.
Materials and Methods
We performed a retrospective, single-center study of all cases of ectopic pregnancies treated medically between January 2014 and December 2018 at the Obstetrics and gynecology department of Military Hospital of Tunis. During these 5 years, 91 patients were diagnosed with ectopic pregnancies. Among these patients, 56 were considered eligible for the medical treatment by Methotrexate.
The inclusion criteria for this treatment regimen were based on Fernandez score:
• HCG level ‹ 5000 mUI/ml
• hematosalpinx diameter ‹ 4 cm
• Absence of significant hemoperitoneum
• Clinical stability
• Absence of acute abdominal pain
• Absence of fetal cardiac activity
Not included were patients with a ruptured ectopic pregnancy, a hemodynamic instability, a low hemoglobin level, an heterotopic pregnancy, a fetal cardiac activity, and corneal, cervical or ovarian ectopic pregnancies. The treatment regimen applied in our study consisted of 1mg of Methotrexate/Kg of body weight (intramuscular injection) that can be administrated a second time if needed. All our patients were admitted on suspicion of ectopic pregnancy, and had a full blood work done: blood type, Complete Blood Count (CBC), test of hemostasis, renal and hepatic tests. Transvaginal ultrasound and HCG assays were done at day 0, day 4 and day 7 to monitor this treatment regimen. The medical treatment was considered successful if HCG level becomes negative without resorting to surgery, even if multiple Methotrexate injections were needed. A second Methotrexate injection was administered if: HCG level at day 7 > day 0 or if it declines less than 15% between day 0 and day 7.If medical treatment is successful, patients are followed with weekly HCG assays until HCG level becomes negative (‹20mUI/ml). If not, the patients required surgical interventions.
Results
During the study period, 91 cases of EP were identified among which 56 were considered eligible for the medical treatment by Methotrexate. The mean age of the patients was 33 years (range 20- 43 years). Mean number of gestations was 3.45 with a maximum of 9 gestations. 53.6% of our patients were nulliparous or primiparous, that made our concern over ulterior fertility stronger. The most common reasons for consultation included vaginal bleeding in 62.5% of cases and abdominal/pelvic pain in 57.1% of cases. 53 patients (94.6%) had amenorrhea when consulting; the amenorrhea duration varied between 4 to 9 weeks. There were no cases of hypovolemic shock. All the patients had a quantitative HCG assay. In 57.1% of cases, HCG level was below 1000 and only 10.7% of cases had a HCG level › 3000. All the patients also had a transvaginal ultrasound. The major sonographic finding is uterine vacuity (91% of cases). A pseudo gestational sac was found in 3.5% of cases. Endometrial thickness was found in 76.7% of cases. A latero uterine mass was found in 32.1% of cases. 40.9% of our patients had a low abundance hemoperitoneum. Fernandez score (Table 1) was the main criteria followed to decide of the medical treatment. For a score ‹13: Medical treatment For a score › 13: Surgical treatment 3 patients presented transitory side effects after receiving a Methotrexate injection; vomiting and abdominal cramps. The medical treatment is considered successful if HCG level becomes negative without needing a surgical intervention (even if we recur to two Methotrexate injections). A second injection of Methotrexate was needed in 35.7% of cases, divided in 3 situations.
1
2
3
Gestational age (DA)
>49
<=49
<=42
hCG level (mUI/ml)
<=1000
1000-5000
>5000
Progesterone level (ng/ml)
<=05
10-May
>10
Abdominal pain
Absent
Provoked
Spontaneous
Hematosalpinx (cm)
<=1
3-Jan
>3
Hemoperitoneum (ml)
<=10
<=100
>100
Table 1: Fernandez scoring system.
• At day 4, HCG level increases by more than 50% compared to day 1 level.
• At day 7, HCG level is superior to day 1 level.
• At day 7, HCG level decreases by less than 15% compared to day 1 level.
After the second injection, the same monitoring is done at day 4 and day 7. If one of these three situations persists, we can say that the medical treatment failed. Medical treatment was successful in 62.5% of cases. Among these successful cases, 85.7% had only one Methotrexate injection. Failure criteria to medical treatment were: a HCG drop below 15% between day 4 and day 7 after the second Methotrexate injection in 52.3% of cases, severe clinical symptoms (acute abdominal pain, hypovolemic shock symptoms, severe anemia) in 18.3% of cases, and the aggravation of ultrasound signs (increase in hemoperitoneum abundance) in 29.4% of cases. 21 patients had a second-line surgery: 15 patients had one MTX injection while 6 had two injections. Laparoscopy was used in 71.4% of cases. A laparotomy was performed in 9.5% of cases while a laparo conversion was needed in 19.1% of cases. All of our patients had a tubal ectopic pregnancy. The exact location of the pregnancy was: the ampulla in 85.7% of cases, the fimbriae in 4.8% of cases and the isthmus in 9.5% of cases. 8 patients had a salpingectomy while 13 had a conservative treatment. The state of the fallopian tube depended essentially on the location of the EP. All the pregnancies that occurred in the fimbriae and the isthmus were ruptured while 44.4% of the ampullary pregnancies were tubal abortions. There were no major post-operative complications.
Discussion
Deciding the treatment regimen for ectopic pregnancies remains tricky. The main challenge is to improve the patient’s quality of life during treatment and to preserve her fertility. In previous Tunisian studies, ectopic pregnancies were treated medically in 27% to 40% of cases. In our study, this percentage is 61.5%, which is even higher than the results reported in the literature [4]. 91 cases of EP were diagnosed in the duration of our study versus 11559 births, which makes its global incidence 0.78%. This incidence is higher than the results found in previous Tunisian studies, with 0.34% in 2005 [5], 0.39% in 2006(3), and 0.6% between 2004 and 2008 [4]. This high incidence is also reported in African literature, ranging between 0.5% and 3.5% [6] and in Occidental literature, ranging between 1% and 2% [7].
This increase in incidence could be explained by the upsurge of multiple risk factors such as sexually transmitted infections, tubal surgeries, use of intrauterine devices, smoking and medically assisted procreation [8,9].Since the founding study of Tanaka in 1982 [10], medical management of ectopic pregnancy using MTX has been developed. Actually, 40 to 50% of ectopic pregnancies are treated by MTX [4,9,11,12]. In our study, the mean age of patients presenting an ectopic pregnancy was 33. In the literature, it ranges between 25 and 35 [6,13,14]. Furthermore, it has been stated that the risk of developing an ectopic pregnancy increases with age. This can be explained by a change in the tubal function with age. Abdominal pain is the most found symptom in patients presenting an ectopic pregnancy, in 74 to 92% of cases [4,15]. However, it was only found in 57.1% of cases in our study (Figure 1). This may be due to early diagnosis. Metrorrhagia was found in 62.5% of cases in our study. However, this frequently found sign is not pathognomonic of an ectopic pregnancy and can be found in miscarriages or threatened abortions. The distinction based on color, abundance and recurrence is difficult to establish [16]. Amenorrhea was found in 94.6% of cases in our study versus 70% in the literature. But this symptom isn’t usually a reason of consultation if not associated with one of the previously described signs [16]. According to CNGOF [7], ectopic pregnancy is highly suspected when HCG level is over 1500 mUI/ml and there is no gestational sac in the transvaginal ultrasound. Below this level, the HCG assay is uninformative and should be repeated after 48 hours; HCG kinetic is much more useful than the level. Transvaginal ultrasound has a specificity of 99% and a sensitivity of 69% [17]. The diagnosis was confirmed by this tool in 89% to 94% of cases according to Stovall and al [11] versus 82% in our study. The sonographic confirmation of ectopic pregnancy is only possible if we identify a latero uterine gestational sac with or without a living embryo or an umbilical vesicle. This sign, however, is only observed in 10% to 20% of case [18]. Other indirect sonographic signs of ectopic pregnancy are uterine vacuity, a nonspecific latero uterine mass, and hemoperitoneum. Management of ectopic pregnancy has greatly progressed during the last years. Medical treatment is particularly interesting in locations with high risk of bleeding, like uterine horns, cervix or uterine scars [19]. Moreover, medical treatment is known to be less costly. Many scores are used to decide the treatment regimen in the case of ectopic pregnancy: Carson and Buster, Elito, Fernandez… However, these scores are not commonly used because progesterone levels are needed and are rarely available. That’s why the modified Fernandez score (see table 2below) is more frequently used.
1
2
3
hCG level (mUI/ml)
<=1000
1000-5000
>5000
Hematosalpinx (cm)
<=1
3-Jan
>3
Hemoperitoneum (ml)
<=10
<=100
>100
Table 2: Modified fernandez scoring system.
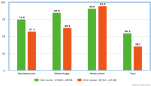
Figure 1: Comparison of symptom frequency between two periods.
For a score ‹7: Medical treatment
For a score ›=7: Surgical treatment
CNGOF recommends in 2003 [7] that medical treatment for ectopic pregnancy should be used if all these criteria are met: HCG‹1000 mUI/ml, pauci-symptomatic pregnancy, and no latero uterine mass. Medical treatment is still recommended if HCG< 5000 mUI/ml and hematosalpinx< 4 cm. Methotrexate is the most used molecule in this situation. It can be used locally or systemically, and is effective on all the ectopic pregnancy locations [20]. It is usually administered by intramuscular injection at the dosage of 1mg/Kg of body weight [21]. There are many possible MTX treatment regimens, with one or multiple injections. In France, the single-dose regimen is the most used, as opposed to the United States [12]. In our study, we used a single-dose regimen with the possibility of a second MTX injection at day 4 or day 7. Medical treatment of ectopic pregnancy should be rigorously monitored. The exacerbation of pelvic pain is commonly seen in the first days following the MTX injection, coinciding with the necrosis or the tubal abortion of the pregnancy. This sign is reported in 30% to 60% of cases in the literature [22] and should be differentiated from the tubal rupture that can occur at any moment after the injection. HCG level is also monitored: it can increase during the 4 first days post injection which can be explained by the initial acceleration of the ectopic pregnancy’s metabolism by the MTX and by the destruction of trophoblastic cells. HCG level should decrease at day 7. The drop of HCG level should be important between day 0 and day 7 or day 4 and day 7 (different recommendations in the literature) as shown in figure 2 below. If not, a second MTX injection or even a laparoscopy may be needed. The effectiveness of medical treatment varies between 65 and 95% in the literature [23]. In our study, the success rate of MTX treatment was 62.5%, similar to the literature. The difference in the success rates in these studies could be explained by the variability of inclusion criteria and the definition of failure. Many studies have confirmed that there is a positive correlation between the initial HCG levels and the success probability of medical treatment. Failures were more reported with HCG level above 1000 mUI/ml for Stika and al [24], 2000 mUI/ml for Sagiv [25], and 5000 for Menon [26]. However, the most used cut-off level currently is 5000. In regard to the number of injections, Barnhart [17] proved that a multi-dose protocol was more effective than a single-dose one, but that it was most costly and more prone to cause adverse effects. The definition of failure of medical treatment of ectopic pregnancy varies according to authors. In our study, failure was when we resorted to surgical treatment (a second MTX injection was not considered a failure), and was reported in 37.5%. The most reported causes of failures are acute abdominal pain, the increase of hemoperitoneum abundance, suspecting a tubal rupture, an unfavorable evolution of HCG levels, and the patient’s refusal of getting another MTX injection [27]. Methotrexate can cause multiple adverse effects like stomatitis, colitis, nausea, abdominal pain, vomiting, leukopenia, and transitory hepatic enzymes elevation [28]. These complications are dose-dependent, and are seen in 61% of cases in multi-dose regimen and in 5 to 24% of cases in singledose regimen [29]. In our study, adverse effects were reported in 31% of cases. The effect of MTX on ulterior fertility is hard to evaluate because many patients are lost to follow-up and/or don’t want to get pregnant. Pregnancy rate after an ectopic pregnancy is 60%, with a recurrence rate of 10% to 30% [7]. Studies have shown that ulterior fertility is comparable after medical and surgical treatment of ectopic pregnancy. However, the recurrence risk is lower when MTX is used. In our study, spontaneous pregnancy rate was 53% with 7.1% recurrent ectopic pregnancy (during 3 years of observation): our results are close to those found in the literature.

Figure 2: HCG levels after Medical treatment.
Conclusion
Our study confirms that medical treatment of ectopic pregnancies by Methotrexate is a legitimate therapeutic option that can be an alternative to laparoscopy, which is still the gold standard in this situation. It represents a low cost and effective alternative, and ulterior fertility is preserved in most cases. The success of medical treatment depends essentially on the education of patients who need to come to the hospital as soon as the symptoms appear, and on the training of medical and paramedical staff who need to always suspect an ectopic pregnancy when a sexually active woman seeks medical attention. The lack of knowledge of this condition often leads to a delay in diagnosis, which can affect the patient’s ulterior fertility and even be life threatening.
References
- Bouyer J. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. 2002; 17: 3224-3230.
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. The Lancet. 2006; 367: 1066-1074.
- Ayadi S. Le traitement médical de la grossesse extra-utérine par méthotrexate: Etude à propos de 83 cas. [Tunis]. 2006.
- Mliki. Traitement médical de la grossesse extra utérine: à propos de 33 cas. [Tunis]. 2009.
- Louati G. Traitement médical de la grossesse extra-utérine: Etude à propos de 52 cas. [Sfax]. 2005.
- Randriambololona DMA, Anjaharisoaniaina NT, Rekoronirina EB, Harioly MOJ, Randriambelomanana JA, Andrianampanalinarivo RH. Ectopic pregnancy in Madagascar: 107 cases. Médecine Santé Trop. 2012; 22: 394-397.
- CNGOF. Collège National des Gynécologues et Obstétriciens Français. [Guidelines for clinical practice: Ectopic pregnancy management]. J GynecolObstetBiolReprod (Paris). 2003; 32: 3S6-112.
- El Younsi A. Aspect thérapeutique de la grossesse extra utérine. [Marrakech]; 2009.
- Fadhlaoui A, Oueslati H, Khedhiri Z, Khrouf M, Chaker A, Zhioua F. [Cost of medical treatment with methotrexate for ectopic pregnancy. Study comparing medical treatment versus laparoscopy. Experience of Aziza Othmana Hospital]. Tunis Med. 2013; 91: 112-116.
- Tanaka T, Hayashi H, Kutsuzawa T, Fujimoto S, Ichinoe K. Treatment of interstitial ectopic pregnancy with methotrexate: report of a successful case. FertilSteril.1982; 37: 851-852.
- Stovall TG, Ling FW. Ectopic pregnancy. Diagnostic and therapeutic algorithms minimizing surgical intervention. J Reprod Med.1993; 38: 807-812.
- Huynh Ngoc Bao Tran. Méthotrexate et grossesses extra utérines. [Limoges]. 2007.
- Amqrane F. La prise en charge de la grossesse extra-utérine à l’hôpital de Kénitra. [Rabat]; 2016.
- Iqraoun S. La grossesse extra-utérine au service de gynéco obstétrique II: à propos de 161 cas. [Fès]. 2016.
- Sy T, Diallo Y, Toure A, Diallo FB, Balde AA, Hyjazi Y, et al. [Management of ectopic pregnancy in Conakry, Guinea]. Med Trop Rev Corps Sante Colon. 2009; 69: 565-568.
- Dupuis O, Clerc J, Madelenat P, Golfier F, Raudrant D. Grossesse extrautérine. Encycl. Méd. Chirurg. Gynécologie obstétrique. 2009; 5-032.
- Barnhart K, Gosman G, Ashby R, Sammel M.. The medical management of ectopic pregnancy: a meta-analysis comparing “single dose” and “multidose” regimens. ObstetGynecol. 2003; 101: 778-784.
- Poncelet é, Leconte C, Fréart-Martinez é, Laurent N, Lernout M, Bigot J, et al. Aspect échographique et IRM de la grossesse extra-utérine. Imag Femme. 2009; 19: 171-178.
- Jermy K, Thomas J, Doo A, Bourne T. The conservative management of interstitial pregnancy: THE CONSERVATIVE MANAGEMENT OF INTERSTITIAL PREGNANCY. BJOG Int J ObstetGynaecol. 2004; 111: 1283-1288.
- Rongières C. Grossesse extra-utérine : pour le traitement conservateur médical. Gynécologie Obstétrique Fertil. 2007; 35: 67-69.
- Gervaise A, Fernandez H. Prise en charge diagnostique et thérapeutique des grossesses extra-utérines. J GynécologieObstétriqueBiolReprod. 2010; 39: F17-F24.
- Nowak-Markwitz E, Michalak M, Olejnik M, Spaczynski M. Cutoff value of human chorionic gonadotropin in relation to the number of methotrexate cycles in the successful treatment of ectopic pregnancy. FertilSteril. 2009; 92: 1203-1207.
- Ozyuncu O, Tanacan A, Duru S, Beksac M. Methotrexate Therapy for Ectopic Pregnancies: A Tertiary Center Experience. Rev Bras Ginecol E Obstetrícia RBGO Gynecol Obstet. 2018; 40: 680-685.
- Stika CS, Anderson L, Frederiksen MC. Single-dose methotrexate for the treatment of ectopic pregnancy: Northwestern Memorial Hospital three-year experience. Am J Obstet Gynecol. 1996; 174: 1840-1848.
- Sagiv R, Debby A, Feit H, Cohen-Sacher B, Keidar R, Golan A. The optimal cutoff serum level of human chorionic gonadotropin for efficacy of methotrexate treatment in women with extrauterine pregnancy. Int J Gynecol Obstet. 2012; 116: 101-104.
- Menon S, Colins J, Barnhart KT. Establishing a human chorionic gonadotropin cutoff to guide methotrexate treatment of ectopic pregnancy: a systematic review. FertilSteril. 2007; 87: 481-484.
- Lee JH, Kim S, Lee I, Yun J, Yun BH, Choi YS, et al. A risk prediction model for medical treatment failure in tubal pregnancy. Eur J ObstetGynecolReprodBiol. 2018; 225: 148-154.
- Gervaise A. Traitement ambulatoire de la grossesse extra-utérine: Alternatives à l’hospitalisation en gynécologie obstétrique. La Lettre du gynécologue. 2005; 301: 31-34.
- Tug N, Sargin MA, Yassa M. Multidose Methotrexate Treatment of Ectopic Pregnancies with High initial β-Human Chorionic Gonadotropin: Can Success Be Predicted? GynecolObstet Invest. 2019; 84: 56-63.