Abstract
Purpose: The current study hoped to explore the optimal exercise modality for overweight or obese patients with type 2 diabetes mellitus combined with depression in conjunction with salivary cortisol monitoring. The goal was to improve patients’ general well-being through exercise, promote weight loss, and better control blood glucose levels.
Method: Patients (BMI=25kg/m2) with T2DM aged between 65 and 75 years were recruited into the study if Hamilton Depression Rating Scale 17 (HDRS-17) score was=8. Participants were randomly assigned to four groups: a control group, an aerobic group (AEX), a resistance exercise group (REX), and a combination exercise group (COMB), which took place 3x/week for 12 weeks. Primary outcome was depression severity as assessed with the HDRS-17, BDI and PSDQ. FBG and HbA1c were assessed as secondary outcomes.
Result: The severity of depressive symptoms was significantly reduced in all exercise intervention groups during the treatment period. The time vs. group interaction for HDRS -17 and BDI was significant in all three exercise intervention groups. However, only the time-to-group interaction for the mean PSDQ endurance subscale was significantly different in the REX and COMB groups compared to the CON group. All exercise groups experienced significant reductions in FBG levels (p<0.05), with the COMB group showing the largest decrease (-1.4±0.6 mmol/l).
Conclusion: Therefore, adopting a lifestyle that combines aerobic and resistance exercise is a recommended health strategy for overweight or obese patients with T2DM combined with depression.
Keywords: type 2 diabetes mellitus; Exercise; Depression; Obese; Hypothalamic-pituitary-adrenal (HPA) axis
Introduction
Individuals with Type 2 Diabetes Mellitus (T2DM) have twice the risk of depression as non-diabetics. This may be due to the physical and psychological stresses associated with T2DM, such as long-term blood glucose management, possible complications, and impact on quality of life [1,2]. Similarly, people with depression have an increased risk of developing T2DM, about 1.5 times that of the general population [3]. This may be because people with depression tend to have poor lifestyle habits, such as lack of exercise, unhealthy eating habits, and sleep disorders, all of which may increase the risk of developing T2DM [4]. For patients with both T2DM and depression, a comprehensive management strategy is needed, including pharmacotherapy, psychotherapy, and lifestyle interventions. These should aim to control blood glucose levels and improve mental health simultaneously. Obesity and lack of physical activity are common risk factors for T2DM and depression [5]. Overweight and obese contribute to the development of cardiovascular disease, cancer, T2DM, hypertension, dyslipidemia, and mental health disorders, which often stem from sedentary lifestyles and excessive energy intake [6,7]. A common mechanism for these problems may be that hyperactivation of the HPA axis and sympathetic nervous system overreaction are common in T2DM, depression, and cognitive dysfunction. Chronically elevated cortisol levels (hypercortisolism) further increase the risk of metabolic syndrome, which is characterized by central obesity, excessive accumulation of abdominal fat, and insulin resistance [8]. Elevated cortisol levels also increase the risk of diabetes [9]. This set of problems creates a vicious cycle that makes it more difficult for people with depression to adhere to healthful behaviors such as healthy eating and exercise, which further increases the risk of developing diabetes. Therefore, Exercise is considered an important component of lifestyle management for overweight or obese patients with type 2 diabetes mellitus combined with depression, as it can help improve blood sugar control, reduce body weight, increase insulin sensitivity, and improve cardiovascular health. Combined exercise, specifically aerobic and resistance exercise, is often recommended for T2DM patients [10].
However, studies on the efficacy of different exercises on depression have reached different conclusions [11]. Future studies should further explore the specific effects of different exercise types, intensities, and durations on patients with depression and the relationship between these changes and the relief of depressive symptoms. Attention should also be paid to individual differences to develop personalized exercise prescriptions for different patients.
Physical activity, especially targeting the Hypothalamic-Pituitary-Adrenal (HPA) axis regulation, has been widely recognized as one of the important non-pharmacological interventions to promote physical and mental health [12] [13]. According to existing health guidelines, at least 150 minutes of moderate to vigorous aerobic exercise per week is highly recommended for adults with diabetes. These forms of exercise can be varied to meet the interests and needs of different patients, such as brisk walking, bicycling, swimming or dancing [14].
Tai Chi, a physical and mental workout that originated in China and has become popular worldwide, offers an ideal workout option for people of all ages with its low to moderate intensity. For people with T2DM, Tai Chi demonstrates unique health benefits [15]. In several dimensions regarding health benefits, Tai Chi demonstrates similarities with common exercise modalities. In addition, Tai Chi improves self-perceived health, helping patients develop a more positive attitude toward life and health [16].
Much of the past research has focused on the effects of aerobic exercise training on depressive symptoms, while resistance exercise training has received relatively little research in this area. However, research in recent years has begun to reveal the potential benefits of resistance exercise training on mental health, including depressive symptoms [17,18]. Recent meta-analyses have shown that resistance exercise training (REX) significantly reduces depressive symptoms in healthy adults and clinically depressed patients [19].
The current study hoped to explore the optimal exercise modality for overweight or obese patients with type 2 diabetes mellitus combined with depression in conjunction with salivary cortisol monitoring. The goal was to improve patients' general well-being through exercise, promote weight loss, and better control blood glucose levels.
Methods
Study Participants
One hundred adults with T2DM who were sufficiently active and physically fit were recruited (age 65-75 years, BMI=25kg/m2). Inclusion criteria were defined as any patient with a mild to moderate depressive episode scoring at least eight on the 17-item Hamilton Depression Scale (HDRS17; 8 points represent mild depression severity) [20]. Patients were randomized to 12 weeks of four groups: a control group, an aerobic group (AEX), a resistance exercise group (REX), and a combination exercise group (COMB).
The exclusion criteria included: (a) Presence of a somatic condition that does not permit regular physical activity (AE). (b) Body mass index (BMI) = 35 kg/m². (c) Being pregnant at baseline. (d) Acute suicidal ideation. (d) Co-morbid severe mental disorders. (e) Regular participation in high-intensity sports activities.
All participants signed an informed consent, and the Institutional Review Boards of all participating institutions approved the study (2024-013-01).
Study Design
The intervention method was conducted as follows.
The control group received regular diabetes treatment, including diet amendments, regular and balanced exercise, and abstinence from smoking and alcohol. According to their individual condition, metformin tablets or subcutaneous injections of insulin aspartate were delivered to control blood sugar in the required range. For participants with hypertension and hyperlipidemia, this was combined with valsartan, atorvastatin, and other drugs to control blood pressure and blood lipid levels in normal ranges. All depressed patients received standard treatment, including medication, individual psychotherapy, and group psychotherapy.
Each exercise intervention group attended exercise sessions three times per week, lasting 60–90 minutes, with 15 minutes of warm-up and cool-down included.
We standardized the aerobic exercise prescription based on body weights of 41.8 (10) kilojoules (kcal) (COMB) and 50.2 (12) kilojoules (kcal) (AER) kg body weight–1 week–1 of moderate exercise. In the COMB group, participants were asked to complete resistance training twice per week, consisting of one set of strength-training maneuvers per set and 41.8 (10) kJ (kcal) kg body weight-1 (week-1) burned through aerobic exercise. Elastic band training exercises include chest clamps, deep Squat Push-Ups, and elastic band standing lateral flexion.
We used the Borg Physical Exertion Scale to estimate participant fatigue and maintained the intensity of each exercise at a level of 3-5. All exercise sessions were supervised by exercise physiologists at our facility. During monthly visits, the control group attended educational sessions about a healthy diet and was asked not to participate in external weight loss or exercise programs. Age, Gender, Smoking Status and Educational Background were usually recorded by means of semi-structured clinical interviews. All patients underwent a thorough physical examination including Electrocardiogram (ECG), Blood Pressure (BP), resting heart rate, weight and height measurements. Body Mass Index (BMI) is determined by the formula weight (kg)/height (m)2. After the HDRS17 scores were completed by trained staff, all patients were asked to complete the 21-item Beck Depression Inventory (BDI) scale [21] and return it the same day or the next. The Physical Self-Description Questionnaire (PSDQ) assessed patients' exercise capacity, self-esteem, and health [22].
In addition, we assessed the HDRS17, BDI, and PSDQ at weeks 4 and 8 (during the intervention) to detect dynamic changes in depressive symptoms. Blood measurements included Fasting Blood Glucose (FBG), fasting C-peptide, fasting insulin, glycosylated Hemoglobin (HbA1c), Total Cholesterol (TC), Triglycerides (TG), Low-Density Lipoprotein Cholesterol (LDL-C) , Hhigh-Density Lipoprotein Cholesterol (HDL-C), Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Uric Acid (UA), Creatinine (Cr), Urea Nitrogen (BUN).
Participants self-collected samples using a saliva collection device in their homes at six-time points during the day: upon waking, 15, 30, and 60 minutes after waking, at 12:00 noon, and at 8:00 p.m.
Using the cortisol concentration values at these four time points 0, 15, 30, and 60 min after awakening, the researchers calculated the increase in cortisol in response to arousal (AUCi). The AUCi reflects the change in cortisol concentration (either an increase or a decrease) in response to arousal and thus is indicative of the responsiveness and sensitivity of the HPA axis to arousal as a stressor. To assess the total activity of the HPA axis throughout the day, the investigators chose values of cortisol concentration at three time points (at awakening, at noon, and at 8:00 p.m.). They calculated the area under the curve (AUC) for daytime cortisol. For the calculation of AUC, we used the trapezoidal formula introduced by Pruessner et al [23].
Statistical Analysis
IBM SPSS 26.0 was applied for data processing. Measurement data were described as the mean ± standard deviation. Counting data was described by frequency and percentage. Multiple group comparisons were analyzed using Analysis of Covariance (ANCOVA). Changes in baseline were treated as dependent variables, while group, visit time, and the interaction of group and visit time (group × visit) were treated as independent effects. In addition, baseline values and sex were treated as covariates. The comparison of the differences between the groups was tested by the Tukey test. Differences were considered statistically significant at P<0.05.
Result
As shown in Tables 1 and 2, no significant differences were found at baseline between participants assigned to either the active experimental group or the active control group in terms of the main study variables (including BDI, HDRS17, and PSDQ scores), diagnosis, education, and smoking status. We also achieved a balanced gender distribution (52% female in the total sample). Before the trial, the two groups of subjects were comparable on several important dimensions, providing a solid basis for subsequent assessment of treatment effects. As can be seen in Figure 1 and Table 2, the severity of depressive symptoms was significantly reduced in all exercise intervention groups during the treatment period. The time vs. group interaction for HDRS -17 and BDI was significant in all three exercise intervention groups. However, only the time-to-group interaction for the mean PSDQ endurance subscale was significantly different in the REX and COMB groups compared to the CON group.
Characteristic
CON (n = 25)
AEX (n = 25)
REX (n = 25)
COMB (n = 25)
Age, years
64±7
65±8
66±5
65±6
Sex, number (%)
Male
12 (48)
10 (40)
15 (60)
11 (44)
Female
13 (52)
15 (60)
10 (40)
14 (56)
Smoking status, smoker (%)
8 (32)
5 (20)
9 (36)
6 (24)
Educational background
Compulsory school
6
2
4
5
High school
15
18
17
14
Higher education
4
5
4
6
Data are presented as mean ± standard deviation.
Notes: CON = control; AEX = weight management and aerobic training; REX = weight management and resistance training; COMB = weight management and combined aerobic and resistance training.
Table 1: Baseline Characteristics of the Participants.
Outcome Variables
CON
AEX
REX
COMB
p Value*
Group–Time InteractionAEX vs CON
REX vs CON
AEX vs REX
COMB vs AEX
COMB vs REX
BDI
Baseline
27.8±9.2
28.3±8.5
28.8±8.0
29.2±9.4
0.015
0.021
0.011
0.24
<.001
<.001
Change at 12 weeks
-3.5±3.8
-14.0±8.5†
-12.8±7.1†
-17.9±10.1†
HDRS-17
Baseline
16.7±5.2
16.2±4.8
15.9±4.9
15.7±6.8
0.028
0.016
0.023
0.48
<.001
<.001
Change at 12 weeks
-3.1±2.6
-9.8±5.8†
-8.1±7.4†
-10.5±6.8†
Mean PSDQ endurance subscale
Baseline
2.8±1.3
3.0±1.5
2.9±1.3
2.7±1.2
0.62
0.39
0.036
<.001
<.001
0.031
Change at 12 weeks
0.2±0.1
0.2±0.2
0.8±0.6†
1.3±0.3†
FBG (mmol/l)
Baseline
7.26±1.00
8.08±1.24
7.32±1.13
7.68±0.81
0.033
<.001
<.001
0.16
0.14
0.27
Change at 12 weeks
-0.2±0.3
-1.3±0.6†
-1.0±0.4†
-1.4±0.6†
HbA1C (%)
Baseline
8.27±1.22
8.58±0.95
8.14±1.27
8.63±0.97
<.001
0.12
0.27
0.71
0.016
0.026
Change at 12 weeks
-0.2±0.2
-0.5±0.4†
-0.3±0.2
-1.3±0.4†
HOMA-IR
Baseline
4.8±0.9
5.3±0.7
4.3±0.6
4.9±0.7
0.026
0.34
0.18
0.88
0.59
0.27
Change at 12 weeks
-0.3±0.7
-0.6±0.6
-0.2±0.6
-0.9±0.6
BMI (kg/m2)
Baseline
25.9±5.7
26.6±4.4
25.1±6.0
26.0±6.3
0.37
0.02
<.001
0.48
0.27
0.51
Change at 12 weeks
-1.5±3.3
-4.1±3.1†
-5.3±1.7†
-5.3±2.2†
Systolic BP (mmHg)
Baseline
125.9±4.0
121.5±5.8
124.7±6.9
122.9±3.5
0.58
0.11
0.92
0.44
0.49
0.23
Change at 12 weeks
-1.1±4.4
-2.5±2.0
2.6±3.1
1.7±3.2
Diastolic BP (mmHg)
Baseline
83.6±3.8
79.3±3.2
80.9±3.6
82.3±2.1
0.77
0.78
0.38
0.21
0.55
0.11
Change at 12 weeks
3.7±1.7
-3.3±0.9
-2.1±1.5
-1.9±1.2
Salivary cortisol
Mean CAR AUCi (nmol min/L)
Baseline
93.61±199.85
85.94±201.99
89.45±198.31
95.06±217.63
0.59
0.33
0.85
0.16
<.001
<.001
Change at 12 weeks
-7.1±14.8
-9.6±20.9
-10.5±12.6
-59.1±34.1†
Mean log AUCg (nmol h/L)
Baseline
1.75±0.13
1.69±0.20
1.77±0.19
1.78±0.21
0.73
0.52
0.21
0.64
0.74
0.81
Change at 12 weeks
-0.22±0.08
-0.17±0.03
-0.25±0.05
-0.30±0.04
Notes: BDI = Beck Depression Inventory; HDRS-17 = Hamilton Depression Scale 17; PSDQ = Physical self-description quationnaire; BMI = Body mass index; BP = Blood pressure; HbA1C =glycated hemoglobin; FBG = fasting blood glucose; CON = control exercise; AEX = weight management and aerobic training exercise; REX = weight management and resistance training exercise; COMB = weight management and combined aerobic and resistance training exercise; CAR = cortisol awakening response; AUCi = area under the curve with respect to increase; AUCg = area under the curve with respect to ground.
Plus-minus values for the change scores are the least-squares adjusted means ± SE from the repeated-measures analyses of variance; plus–minus values for the baseline values are the observed means ± SE.
*p Values for the comparison among the groups of changes from baseline to 12 weeks were calculated with the use of ANCOVA (with baseline values and sex as covariates). Secondary analyses included a comparison between the combination group and the control group.
†p < 0.05 for the comparison of the value at the follow-up time with the baseline value within the group, as calculated with the use of mixed-model repeated measures analysis of variance.
Table 2: Biochemical in type 2 diabetic patients after intervention with different exercise patterns.
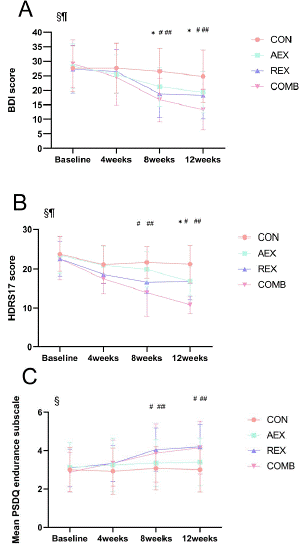
Figure 1: Notes. Scores of (A) BDI, (B) HDRS17 and (C) Mean PSDQ endurance subscale over the intervention with different exercise patterns.
Using two-way ANOVA: § p < 0.05 for the effect of time; ¶ p < 0.05 for the interaction between exercise and time. Using Tukey’s multiple comparison test: *p < 0.05 for the difference between AEX and CON; # p < 0.05 for the difference between REX and CON; ## p < 0.05 for the difference between COMB and CON. Values are means ± SEM.
CON = control exercise; AEX = weight management and aerobic training exercise; REX = weight management and resistance training exercise; COMB = weight management and combined aerobic and resistance training exercise.
The COMB group again demonstrated the most pronounced improvement, with lower HDRS-17 and BDI scores compared to both AEX and REX groups. Fig 1A and Fig 1B show that COMB started to show a significant decrease in HDRS-17 and BDI scores from week 4. Fig 1C shows a significant interaction between exercise modality and time (p < 0.05). This implies that the trend over time was different for different exercise modalities (AEX, REX, COMB); there was a statistically significant difference between REX, COMB, and CON starting at week 8.
All exercise groups experienced significant reductions in FBG levels (p<0.05), with the COMB group showing the largest decrease (-1.4±0.6 mmol/l). Significant HbA1C reductions were observed in AEX, REX, and COMB groups (p<0.05), with the COMB group achieving the greatest decline (-1.3±0.4%). Only the AEX group significantly reduced HOMA-IR at 12 weeks (p<0.05).
All exercise groups, except CON, significantly reduced BMI (p<0.05), with REX and COMB groups achieving the largest decreases (-5.3±1.7 and -5.3±2.2 kg/m2, respectively). While there were no significant changes in systolic BP across groups, REX and COMB groups showed significant reductions in diastolic BP (p<0.05). Only the COMB group demonstrated a significant decrease in salivary cortisol levels, as measured by AUCi (-59.1±34.1 nmol min/L) and AUCg, indicating reduced stress levels.
Discussion
Different exercise modes have significant combined effects on overweight or obese patients with T2DM and depression. Aerobic exercise, resistance exercise, and combined exercise showed positive effects on improving glycemic control, reducing body weight, and alleviating depressive symptoms in patients.
All exercise groups showed positive effects on BMI; a BMI drop has a role in exercise reversing weight loss due to loss of appetite, a common symptom, and a side effect of antidepressants [24]. Depressive symptoms and weight gain are a vicious cycle [25,26]. If healthy behaviors, such as exercise and healthy eating, are adopted, thereby reducing weight gain and improving depressive symptoms, this will improve the quality of life of patients.
All exercise interventions significantly improved BDI scores and HDRS-17 compared to the CON group, with the COMB group showing the most significant improvement (p < .001 for COMB vs AEX and COMB vs REX). Aerobic exercise does affect a variety of physiological mechanisms in the body, including altering levels of monoamine neurotransmitters, increasing levels of 5-HT (serotonin, also known as 5-hydroxytryptophan) and norepinephrine, and decreasing levels of cortisol, which can alleviate symptoms of depression to some extent [24].
Throughout the intervention treatment, although the BDI of all exercise populations changed significantly compared to the control group, we can find that COMB, on the other hand, declined the slowest in the first few weeks but accelerated its decline in the later stages. It may be related to the fact that the patients slowly got used to the combined treatment program in the last stages and accepted this exercise treatment from the inside. After 12 weeks of exercise, HDRS17 scores decreased in all groups, but the COMB group showed the greatest decrease and the most significant improvement. This suggests that a comprehensive exercise program has an optimal positive impact on mood state and quality of life in patients with T2DM combined with depression. Over time, the PSDQ of the COMB group showed the most significant improvement at almost all time points, suggesting that the combined exercise modalities are optimal for enhancing self-perceived fitness in diabetic patients [25].
Combining the antidepressant effects of Tai Chi with the physiological and psychological benefits of resistance exercise may produce a synergistic effect, resulting in a more effective improvement of depressive symptoms. This combined exercise program may improve cardiorespiratory fitness, build muscle strength, promote endorphin release, and reduce stress and anxiety through meditation and relaxation techniques [26].
As an ancient and profound form of physical and mental exercise, Tai Chi uniquely emphasizes the harmony and unity of the body, mind, and external environment [27]. In recent years, with in-depth research on the relationship between mind-body health, more scientific evidence has shown that mind-body exercises such as Tai Chi significantly alleviate depressive symptoms. Depression, as a common mental illness, has symptoms including low mood, loss of interest, fatigue, and weakness, which seriously affect patients' quality of life. Mind-body exercises can effectively alleviate these symptoms and help patients restore their physical and mental health by regulating the body's physiological functions and psychological state [28-30].
Similar to aerobic exercise, resistance exercise may also improve depressive symptoms through mechanisms that affect levels of neurotransmitters such as 5-hydroxytryptamine and norepinephrine and modulate the activity of the hypothalamic-pituitary-adrenal axis [31].
On the other hand, a preliminary trial of a tiny sample of depressed hospitalized patients (n = 12) compared AEX and stretching activities over three weeks. The results showed a significant effect of AEX on depressive symptoms (higher remission rates), in contrast to our findings [32].
Compared to the baseline values in the entire cohort and the three exercise groups, our patients had a significantly reduced FBG level after the 3-month intervention. Compared with patients in the CON, AEX, and REX groups, the COMB group had improved considerably in HbA1c. Previous findings have also shown that diabetes remission is not associated with body mass index or weight loss after exercise interventions [33].
While a single aerobic or resistance exercise can be effective in improving anxiety and helping to control blood sugar and body weight, a prolonged program of high-intensity exercise may increase the rate of lower extremity injuries [34]. Widespread fear of joint damage in overweight and obese patients [35]. Therefore, we believe that the COMB exercise method can be used to achieve the effect of exercise and maximize the protection of joint health. There may have been some differences in salivary cortisol levels across groups at baseline (especially in AUCi without log conversion). Still, after 12 weeks, COMB significantly reduced the AUCi metric. Depressed patients often self-report inadequate levels of physical activity, which may be related to their psychological state and decreased motivation. Under the stress of social evaluation, these patients show a sluggish cortisol response pattern. Cortisol is a stress hormone whose normal response is essential for coping with stress. Elevated serum and salivary cortisol levels in depressed patients are a complex physiologic process involving multiple aspects, such as dysregulation of the HPA axis, effects of intestinal flora, and neurotransmitter interference [36-38]. A sluggish cortisol response may indicate abnormal functioning of the HPA axis in patients, which is an essential aspect of the biological mechanisms of depression [39].
It is essential to increase the level of physical activity in depressed patients systematically. This will not only help improve their physical health but may also positively impact their mental health. Our study suggests that 12 weeks of aerobic or resistance exercise training is not sufficient to affect cortisol stress reactivity in depressed patients significantly. Multiple studies have shown that salivary cortisol levels rise during high-intensity exercise and do not always correlate significantly with stress levels during low- and moderate-intensity training. This phenomenon may be explained by the fact that the body's stress response may be less intense during low- and moderate-intensity training, with little change in cortisol secretion or insufficient change to be detected significantly in saliva. This also explains the results of this experiment [40-42].
Physical activity levels are relatively low in patients with depression. In a previous meta-analysis, Schuch et al. noted that more than two-thirds (68%) of patients with MDD did not meet the current recommended level of physical activity (at least 150 minutes of moderate to vigorous physical activity per week, MVPA) [43]. Our findings did not reveal a significant association between aerobic or resistance exercise interventions alone and patients' cortisol responses, which may be due to the participants' generally low physical activity levels.
Conclusion
Therefore, adopting a lifestyle that combines aerobic and resistance exercise is a recommended health strategy for overweight or obese patients with T2DM combined with depression. It can not only help them improve their physical health but also provide critical support at the psychological level, promoting functional independence and overall well-being.
Author Statements
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
Human and Animal Rights
No animals were used for the study. All human procedures were followed in accordance with the Helsinki Declaration of 1975 as revised in 2013.
References
- Renn BN, Feliciano L, Segal DL. The bidirectional relationship of depression and diabetes: a systematic review. Clin Psychol Rev. 2011; 31: 1239-46.
- Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008; 31: 2383-90.
- Campayo A, de Jonge P, Roy JF, Saz P, de la Camara C, Quintanilla MA, et al. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010; 167: 580-8.
- Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Roux AVD, et al. Examining a bidirectional association between depressive symptoms and diabetes. Jama. 2008; 299: 2751-9.
- Kakoschke N, Zajac IT, Tay J, Luscombe-Marsh ND, Thompson CH, Noakes M, et al. Effects of very low-carbohydrate vs. high-carbohydrate weight loss diets on psychological health in adults with obesity and type 2 diabetes: a 2-year randomized controlled trial. Eur J Nutr. 2021; 60: 4251-4262.
- Sousa RAL, Improta-Caria AC, Souza BSF. Exercise-Linked Irisin: Consequences on Mental and Cardiovascular Health in Type 2 Diabetes. Int J Mol Sci. 2021; 22: 2199.
- Luo X, Zhao M, Zhang Y, Zhang Y. Effects of baduanjin exercise on blood glucose, depression and anxiety among patients with type II diabetes and emotional disorders: A meta-analysis. Complement Ther Clin Pract. 2023; 50: 101702.
- Steffensen C, Dekkers OM, LyhneJ, Pedersen BG, Rasmussen F, Rungby J, et al. Hypercortisolism in Newly Diagnosed Type 2 Diabetes: A Prospective Study of 384 Newly Diagnosed Patients. Horm Metab Res. 2019; 51: 62-68.
- Badescu SV, Tataru C, Kobylinska L, Georgescu EL, Zahiu DM, Zagrean AM, et al. The association between Diabetes mellitus and Depression. J Med Life. 2016; 9: 120-5.
- Chang S, Liu H. Effects of combined resistance training and Tai Chi on oxidative stress, blood glucose and lipid metabolism and quality of life in elderly patients with type 2 diabetes mellitus. Res Sports Med. 2024; 32: 871-884.
- Noetel M, Sanders T, Gallardo-Gomez D, Taylor P, Cruz BDP, van den Hoek, et al. Effect of exercise for depression: systematic review and network meta-analysis of randomised controlled trials. Bmj. 2024; 384: e075847.
- Athanasiou N, Bogdanis GC, Mastorakos G. Endocrine responses of the stress system to different types of exercise. Rev Endocr Metab Disord. 2023; 24: 251-266.
- Stranahan AM, Lee K, Mattson MP. Central mechanisms of HPA axis regulation by voluntary exercise. Neuromolecular Med. 2008; 10: 118-27.
- Thompson PD, Arena R, Riebe D, Pescatello LS, America College of Sports Medicine. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep. 2013; 12: 215-7.
- Lan C, Chen SY, Lai JS, Wong AMK. Tai chi chuan in medicine and health promotion. Evid Based Complement Alternat Med. 2013; 2013: 502131.
- Cocchiara RA, Dorelli B, Gholamalishahi S, Longo W, Musumeci E, Mannocci A, et al. Tai Chi and Workplace Wellness for Health Care Workers: A Systematic Review. Int J Environ Res Public Health. 2020; 17: 343.
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011; 43: 1334-59.
- Netz Y. Is the Comparison between Exercise and Pharmacologic Treatment of Depression in the Clinical Practice Guideline of the American College of Physicians Evidence-Based? Front Pharmacol. 2017; 8: 257.
- Gordon BR, McDowell CP, Hallgren M, Meyer JD, Lyons M, Herring MP. Association of Efficacy of Resistance Exercise Training With Depressive Symptoms: Meta-analysis and Meta-regression Analysis of Randomized Clinical Trials. JAMA Psychiatry. 2018; 75: 566-576.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960; 23: 56-62.
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J An inventory for measuring depression. Arch Gen Psychiatry. 1961; 4: 561-71.
- Marsh HW. Physical Self Description Questionnaire: stability and discriminant validity. Res Q Exerc Sport. 1996; 67: 249-64.
- Pruessner JC, Kirschbaum C, Meinlschmid G, Helhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003; 28: 916-31.
- Nieuwenhuijsen K, Faber B, Verbeek JH, Neumeyer-Gromen A, Hees HL, Verhoeven AC, et al. Interventions to improve return to work in depressed people. Cochrane Database Syst Rev. 2020; 10: Cd006237.
- D’Alleva M, Vaccari F, Graniero F, Giovanelli N, Floreani M, Fiori F, et al. Effects of 12-week combined training versus high intensity interval training on cardiorespiratory fitness, body composition and fat metabolism in obese male adults. J Exerc Sci Fit. 2023; 21: 193-201.
- O’Donoghue G, Blake C, Cunningham C, Lennon O, Perrotta C. What exercise prescription is optimal to improve body composition and cardiorespiratory fitness in adults living with obesity? A network meta-analysis. Obes Rev. 2021; 22: e13137.
- Niño A, Villa-Vicente JG, Collado PS. Functional Capacity of Tai Chi-Practicing Elderly People. Int J Environ Res Public Health, 2022. 19: 2178.
- Chang YK, Nien YH, Tsai CL, Etnier JL. Physical activity and cognition in older adults: the potential of Tai Chi Chuan. J Aging Phys Act. 2010; 18: 451-72.
- Kinser PA, Goehler LE, Taylor AG. How might yoga help depression? A neurobiological perspective. Explore (NY). 2012; 8: 118-26.
- Luo S, Mei Z, Fang G, Mu G, Zhang X, Luo S. Effects of mind-body therapies on depression among adolescents: a systematic review and network meta-analysis. Front Public Health. 2024; 12: 1431062.
- Gordon BR, Mc Dowell CP, Lyons M, Herring MP. The Effects of Resistance Exercise Training on Anxiety: A Meta-Analysis and Meta-Regression Analysis of Randomized Controlled Trials. Sports Med. 2017; 47: 2521-2532.
- Shachar-Malach T, Kazaz RC, Constantini N, Lifschytz T, Lerer B. Effectiveness of Aerobic Exercise as an Augmentation Therapy for Inpatients with Major Depressive Disorder: A Preliminary Randomized Controlled Trial. Isr J Psychiatry Relat Sci. 2015; 52: 65-70.
- Gong Q, Zhang P, Wang J, Ma J, An Y, Chen Y, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019; 7: 452-461.
- Rynecki ND, Siracuse BL, Ippolito JA, Beebe KS. Injuries sustained during high intensity interval training: are modern fitness trends contributing to increased injury rates? J Sports Med Phys Fitness. 2019; 59: 1206-1212.
- Hamer O, Larkin D, Relph N, Dey P. Fear-related barriers to physical activity among adults with overweight and obesity: A narrative synthesis scoping review. Obes Rev. 2021; 22: e13307.
- Hinkelmann K, Moritz S, Botzenhardt J, Muhtz C, Wiedemann K, Kellner M, et al. Changes in cortisol secretion during antidepressive treatment and cognitive improvement in patients with major depression: a longitudinal study. Psychoneuroendocrinology. 2012; 37: 685-92.
- Dziurkowska E, Wesolowski M, Dziurkowski M. Salivary cortisol in women with major depressive disorder under selective serotonin reuptake inhibitors therapy. Arch Womens Ment Health. 2013; 16: 139-47.
- Beck J, Bruni N, Brand S, Holsboer-Trachsler E. Repeated Cortisol Awakening Response as Predictor of Antidepressant Treatment Outcome with Duloxetine. Neuropsychobiology. 2015; 71: 97-102.
- Mikulska J, Juszczyk G, Gawronska-Grzywacz M, Herbet M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021; 11: 1298.
- Duclos M, Guinot M, Le Bouc Y. Cortisol and GH: odd and controversial ideas. Appl Physiol Nutr Metab. 2007; 32: 895-903.
- Mishica C, Kyrolainen H, Hynynen E, Nummela A, Holmberg HC, Linnamo V. Relationships between Heart Rate Variability, Sleep Duration, Cortisol and Physical Training in Young Athletes. J Sports Sci Med. 2021; 20: 778-788.
- VanBruggen MD, Hackney AC, McMurray RG, Ondark KS. The relationship between serum and salivary cortisol levels in response to different intensities of exercise. Int J Sports Physiol Perform. 2011; 6: 396-407.
- Schuch F, Vancampfort D, Firth J, Rosenbaum S, Ward P, Reichert T, et al. Physical activity and sedentary behavior in people with major depressive disorder: A systematic review and meta-analysis. J Affect Disord. 2017; 210: 139-150.
