
Research Article
Austin J Cerebrovasc Dis & Stroke. 2016; 3(1): 1043.
A Prospective Safety Trial of Atorvastatin Treatment to Assess Rebleeding after Spontaneous Intracerebral Hemorrhage: A Serial MRI Investigation
Knight RA1,3*#, Nagaraja TN2#, Li L1#, Jiang Q¹, Tundo K², Chopp M¹ and Seyfried DM²
¹Departments of Neurology, Henry Ford Hospital, USA
²Departments of Neurosurgery, Henry Ford Hospital, USA
³Department of Physics, Oakland University, Rochester, USA
*Corresponding author: Knight RA, Department of Neurology-NMR Research, Henry Ford Hospital, 2799 West Grand Blvd., Detroit, MI 48202, USA
Received: June 15, 2016; Accepted: July 18, 2016; Published: July 20, 2016
Abstract
Aim: This study was designed to determine any rebleeding after atorvastatin treatment following spontaneous intracerebral hemorrhage (ICH) in a prospective safety trial.
Patients: Atorvastatin (80 mg/day) therapy was initiated in 6 patients with primary ICH with admission Glasgow Coma Score (GCS) >5 within 24 hours of ictus and continued for 7 days, with the dose tapered and treatment terminated over the next 5 days. Patients were studied longitudinally by multiparametric magnetic resonance imaging (MRI) at three time points: acute (3 to 5 days), subacute (4 to 6 weeks) and chronic (3 to 4 months). Imaging sequences included T1, T2-weighted imaging (T2WI), diffusion tensor imaging (DTI) and contrast-enhanced MRI measures of cerebral perfusion, blood volume and blood-brain barrier (BBB) permeability. Susceptibility weighted imaging (SWI) was used to identify primary ICH and to check for secondary rebleeding. Final outcome was assessed using Glasgow Outcome Score (GOS) at 3-4 months.
Results: Mean admission GCS was 13.2±4.0 and mean GOS at 3 months was 4.5±0.6. Hemorrhagic lesions were segmented into core and rim areas. Mean lesion volumes decreased significantly between the acute and chronic study time points (p=0.008). Average ipsilateral hemispheric tissue loss at 3 to 4 months was 11.4±4.6 cm3. MRI showed acutely reduced CBF (p=0.004) and CBV (p=0.002) in the rim, followed by steady normalization. Apparent diffusion coefficient of water (ADC) in the rim demonstrated no alterations at any of the time points (p>0.2). The T2 values were significantly elevated in the rim acutely (p=0.02), but later returned to baseline. The ICH core showed sustained low CBF and CBV values concurrent with a small reduction in ADC acutely, but significant ADC elevation at the end suggestive of irreversible injury.
Conclusion: Despite the presence of a small, probably permanent, cerebral lesion in the ICH core, no patients exhibited post-treatment rebleeding. These data suggest that larger, Phase 2 trials are warranted to establish long term clinical safety of atorvastatin in spontaneous ICH.
Keywords: Atorvastatin; MRI; Intracerebral hemorrhage; Rebleeding
Abbreviations
ICH: Intracerebral Hemorrhage; GCS: Glasgow Coma Score; MRI: Magnetic Resonance Imaging; T2WI: T2-Weighted Imaging; DTI: Diffusion Tensor Imaging; BBB: Blood-Brain Barrier; SWI: Susceptibility-Weighted Imaging; GOS: Glasgow Outcome Score; CBF: Cerebral Blood Flow; CBV: Cerebral Blood Volume; ADC: Apparent Diffusion Coefficient of Water; HMG-CoA: 3-Hydroxy- 3-Methyl-Glutaryl-Coenzyme A; IRB: Institutional Review Board; FDA: Food and Drug Administration; L-L: Look-Locker; 3D: 3-Dimensional; IRSPGR: Inversion Recovery Spoiled Gradient Recalled Acquisition; TR: Repetition Time; TE: Echo Time; FA: Flip Angle; BW: Band Width; FOV: Field of View; DCE-MRI: Dynamic Contrast Enhanced MRI; FSE: Fast Spin-Echo; EPI: Echo Planar Imaging; SD: Standard Deviation; SPARCL: Stroke Prevention by Aggressive Reduction in Cholesterol Levels; STITCH: Surgical Trial in Traumatic Intra Cerebral Haemorrhage; Ipsil/Contral: Ipsilateral/ Contralateral; NINDS: National Institute for Neurological Disorders and Stroke; NIH: National Institutes of Health
Introduction
Intracerebral hemorrhage (ICH) is a particularly lethal stroke subtype, with first year mortality approaching 50% and survivors often left with severe disabilities [1]. This poor outcome results from direct tissue damage and mass effect caused by the hematoma, and the presence of an ischemic or partially ischemic perihematomal boundary. In addition, the hematoma itself has been reported to induce other early secondary tissue changes including neuronal and glial loss due to apoptosis and inflammation [2], and vasogenic edema caused by damage to the blood-brain barrier (BBB) [1,3]. While spontaneous ICH accounts for 10-20% of all strokes, effective clinical treatments remain elusive.
Statin (3-hydroxy-3-methyl-glutaryl-coenzyme A [HMGCoA] reductase inhibitor) therapy has been reported to modulate endothelial function and preserve blood flow in ischemic tissue [4] and to exert neuroprotection due to pleiotropic effects via upregulation of angiogenesis, neurogenesis and synaptogenesis [5-7]. Statins have also shown therapeutic benefit following experimental ICH [8-16] and in clinical ICH setting [17-23]. However, despite several studies reporting neuroprotective effects of statins in cerebrovascular diseases, reservations persist regarding their clinical use in ICH, with a presumed increased risk of hemorrhage recurrence with statin treatment [24-26].
These circumstances have resulted in uncertainty regarding statin therapy in acute ICH [27-28]. However, with virtually no other effective therapies available for this extremely debilitating and high mortality condition, trials in humans are urgently needed to comprehensively test the efficacy of statins [29]. This prospective, nonrandomized trial evaluated the safety of statin treatment with respect to hemorrhage recurrence after primary ICH event in a patient cohort with an admission GCS >5. In addition to clinical evaluations, patients also underwent serial MRI investigations to assess ICH-induced changes in cerebral blood flow and volume, BBB permeability, edema and hemorrhage recurrence at acute, sub-acute and chronic time points.
Materials and Methods
Patient selection and treatment
Atorvastatin (80 mg/day) therapy was initiated in ICH patients (mean age 52.7 ± 12.4 years) with GCS greater than 5 within 24 hours of ictus. Treatment was continued for 7 days, and then tapered off over 5 days (20 mg/day x 3 days followed by 10 mg/day x 2 days). Final neurological outcome was assessed via the GOS at 3 to 4 months. Exclusion criteria included prior statin use, preexisting severe neurological conditions, ICH less than 2 cm maximum diameter, cranial arteriovenous malformations, aneurysm, tumor, trauma, pregnant, elevated CPK or myocardial infarction within 30 days. This study was approved by the Institutional Review Board committee (IRB# 3921).
MRI protocol
Patients were studied longitudinally by MRI at three time points (acute = 3 to 5 days, subacute = 4 to 6 weeks, and chronic = 3 to 4 months). Two of the 6 patients were not studied at the subacute time point. All MRI studies were performed using a 3 Tesla 94 cm bore, Food and Drug Administration (FDA) approved clinical MRI system (GE Medical Systems, Milwaukee, WI) running Excite HD (software version 12.0) with a gradient subsystem capable of producing field gradients of 40 mT/m and 150 T/m/sec slew rate. An 8-channel phased array head coil was used for signal reception. The imaging protocol included multislice T1, T2, flow-compensated susceptibilityweighted imaging (SWI), diffusion-tensor imaging (DTI), and a spiral Look-Locker (L-L) imaging sequence for T1 mapping. Contrast enhancement was used in conjunction with MRI pulse sequences designed to measure changes in cerebral perfusion, blood volume and blood-brain barrier permeability. Dynamic contrast enhancement was produced by injecting 20 ml of Magnevist (gadopentetate dimeglumine; Bayer Healthcare Pharmaceuticals, Wayne, NJ) as an intravenous bolus at a rate of 4 ml/second using an Opti-Star power injector (Mallinckrodt, Hazelwood, MO). The number of slices was adjusted to encompass the entire region of the hematoma and surrounding rim. Imaging parameters for the various sequences are described in the following sections.
Standard MR imaging sequences
At each study time, a set of standard imaging sequences was obtained to evaluate the location, size and paramagnetic characteristics of the hematoma core and rim area over time before collecting the quantitative dynamic contrast enhanced MRI data. First, an SWI sequence was acquired using a 3-dimensional (3D) spoiled gradient recalled acquisition in the steady state (SPGR) sequence with repetition time (TR) = 33 ms, echo time (TE) = 20 ms, flip angle (FA) = 15 degree, bandwidth (BW) = 15.63 kHz, FOV = 24 cm, imaging matrix 512 x 512, 2 mm slice thickness, and 1 average with flow compensation.
Next, a 3D inversion recovery SPGR (3D-IRSPGR) volumetric scan was acquired with TE/TI/TR of 3.5/500/8.8 msec, flip angle 15°, imaging matrix 256 x 192 x 172, 24 cm field of view (FOV), phase FOV 0.75, 1 mm slice thickness. A transverse relaxation (T2) weighted imaging sequence was used to acquire T2-weighted images (T2WI), which were subsequently used to identify and localize the hematoma region and surrounding edematous rim. The images were acquired using a fast multi echo sequence with TR/TE = 2500/30 and 117 ms, imaging matrix = 320 x 224, FOV = 24 cm, and 4 mm slice thickness.
Finally, diffusion tensor imaging (DTI) [30,31] was acquired to determine whether diffusion-weighted imaging could be used to detect post-ICH tissue recovery, particularly of white matter, in adjacent regions bordering the hematoma (the rim). The sequence used an echo-planar imaging (EPI) sequence with TR/TE = 6500/92 ms, field of view = 24 cm, 96 x 96 imaging matrix, slice thickness = 2.6 mm, b-value = 1500 s/mm2, 55 directions, 1 average.
Contrast enhanced MRI sequences
In this study, dynamic contrast-enhanced MRI (DCE-MRI) was used to generate quantitative estimates of cerebral blood flow (CBF), cerebral blood volume (CBV) and pre and post-contrast T1 images to check for contrast enhancement by subtraction. Initial baseline T1-weighted images (T1WI) were obtained using a fast spin-echo (FSE) sequence with TE = 14 ms, TR = 2500 ms, 24 cm FOV, imaging matrix 320 x 224, and 4 mm slice thickness. To obtain a baseline level for localizing BBB permeability, a fast Look-Locker T1-weighted spiral EPI sequence (TR/TE = 1800/5.7 ms, xres of 4096, yres of 4, 24 cm FOV, three 4 mm thick slices) was used to obtain quantitative estimates of T1 prior to and following contrast injection.
Before acquiring the BBB permeability data, DCE-MRI based estimates of CBF and CBV were obtained [32-34]. For measurements of CBF and CBV, a spin-echo EPI sequence was used with 128 x 128 data acquisition matrix, TR/TE = 1900/24.2 ms, 24 cm FOV, to image nine 4 mm thick slices. The DCE cerebral perfusion measurement acquired 99 sets of EPI images; ten sets before Magnevist injection and 89 sets after. After acquiring the CBF/CBV data set, a series of quantitative post-contrast T1 measurements were collected to track the clearance of the contrast agent over time. Six sets of T1 estimates were acquired using the fast Look-Locker T1-weighted spiral EPI sequence described above.
Finally, after collecting the BBB permeability data set, a final postcontrast T1WI data set was obtained using the previously described T1WI FSE sequence. Identification of core and border (rim) regions of interest was performed by thresholding T2 values and estimates of lesion volume (core + rim) and hemispheric tissue loss were measured from T2WI. Measures of T2 and the apparent diffusion coefficient (ADC) were obtained from the T2WI and DTI data sets, respectively. Estimates of CBF and CBV were calculated for each time point using the DCE-MRI data sets with Eigen tool analysis [35]. Brain regions with changes in vascular permeability were identified by subtracting pre-contrast T1 from post-contrast T1 images.
All data are reported as mean ± standard deviation (sd) and were analyzed using Student’s t-tests. Significance was inferred for p ≤ 0.05.
Results
Six patients (3 male and 3 female) with ICH were studied (mean age 52.7 ± 12.4 years). All ICH loci were deep and 3 were left-sided. The mean GCS at admission was 13.2±4.0 and the mean GOS at 3 months was 4.6 ± 0.5. No patients re hemorrhaged during the study and none were dropped due to drug-related complications. Mean lesion volume decreased significantly between the acute and chronic time points (Figure 1). Average ipsilateral hemispheric tissue loss at 3 to 4 months ranged from about 6 cc to 17 cc with a mean of 11.4±4.6 cc.
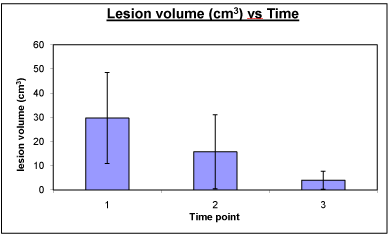
Figure 1: Plot of lesion volumes at acute (1), sub-acute (2) and chronic (3)
time points. The lesion volume decreased steadily across time with both
the core and rim regions decreasing. The decrease in lesion volume was
significant between the acute and chronic time points.
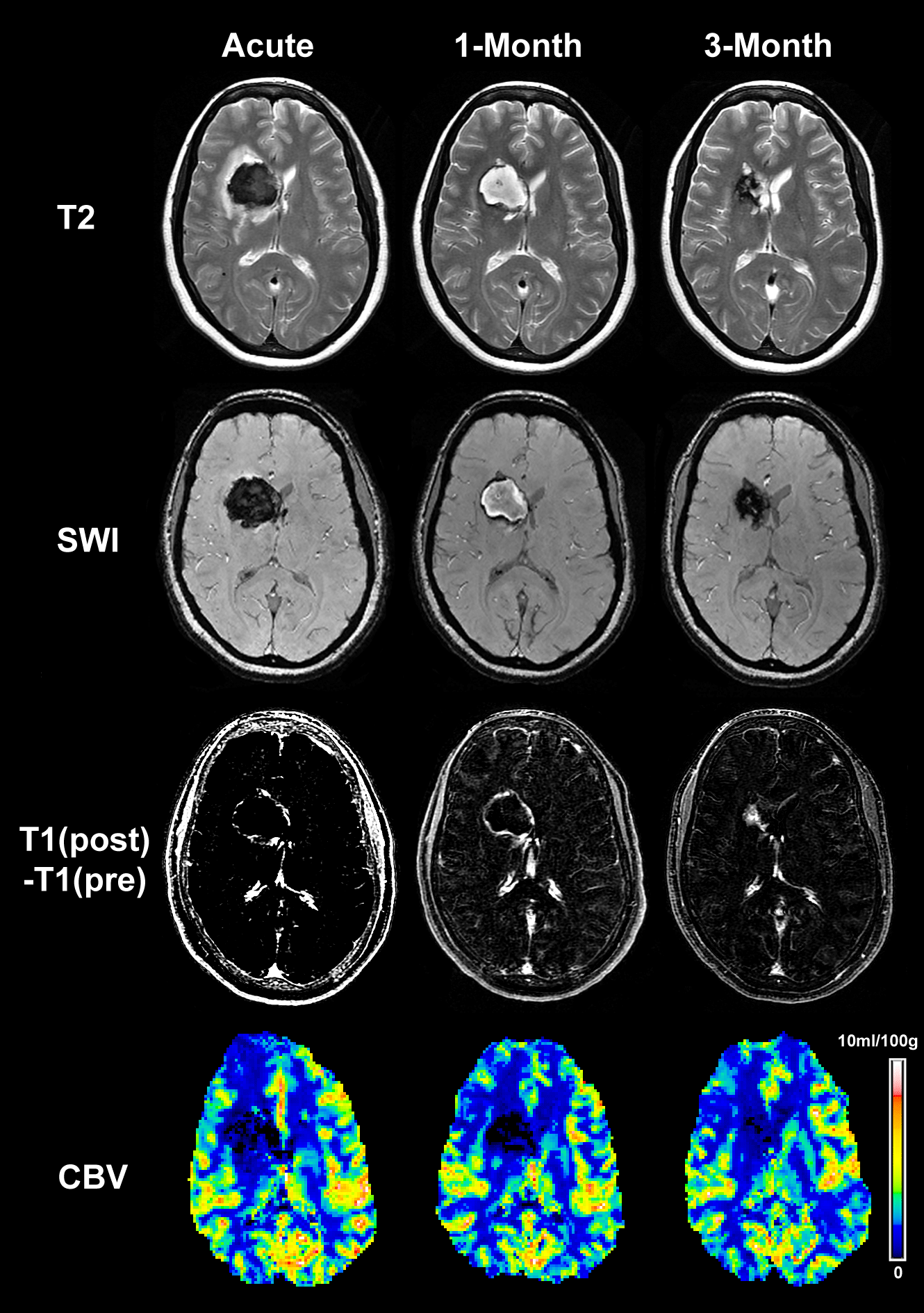
Figure 2: Representative images of a patient with ICH at acute, sub-acute
and chronic time points. The upper row presents T2-weighted images
showing the temporal evolution of the hematoma and peri-hematoma rim,
from which the core and rim regions were identified. The 2nd and 3rd rows
show the corresponding SWI and T1 subtraction images, respectively. Finally,
the bottom row presents the CBV maps from the 3 time points. There seemed
to be some persistent CBV reductions, probably reflecting secondary effects
of the ICH. It can be noted that no secondary post-ICH rebleeding was seen
by SWI. The extent of the peri-hematoma rim acutely can be appreciated by
visual comparison of the SWI and T2WI.
The T2WI and SWI sequences provided excellent discrimination of the ICH core and rim areas at all time points studied. A temporal series of T2WI, SWI, T1 subtraction and CBV images from a representative ICH patient obtained at acute, sub-acute and chronic time points are shown in Figure 2. Dramatic shifts in T2 signal intensity were seen in the core and rim areas between acute and sub-acute time points. Average T2±SD values are shown plotted as a function of time in Figure 3. The T2 values were significantly elevated in the rim area acutely (p=0.02), but returned to baseline at the subacute and chronic time points. In the core, T2 values decreased acutely, increased subacutely, and then declined again at the chronic time with the changes differing significantly from contralateral levels at all three times.
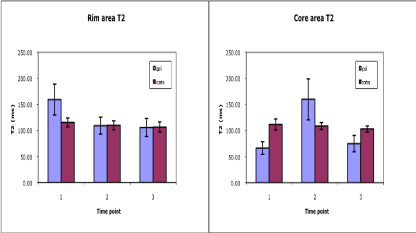
Figure 3: Average T2 values plotted as a function of time post-ICH. T2 values for the adjacent rim (left) and the central core (right) regions of interest located in the
ipsilateral and corresponding contralateral hemispheres are shown at acute (1), sub-acute (2) and chronic (3) time points. The low T2 values in the core at acute
and chronic time points probably is due to the presence of large amounts of deoxyhemoglobin and hemosiderin, respectively, whereas the elevated T2 subacutely
probably reflects edema. In the rim region, the acute T2 elevation was probably due to edema.
Temporal changes in CBF for the core and rim areas are shown in Figure 4. Estimates of CBV obtained from the same MRI data sets closely tracked with CBF changes and are not shown. MRI revealed significant reductions in CBF (p=0.004) and CBV (p=0.002), relative to contralateral ROI values, in the ICH rim area acutely that improved with time. The initial low CBF and CBV levels measured in the rim were followed by steady normalization at the subacute and chronic time points with significant improvement in CBV (p=0.035) and marginally significant improvement (p=0.055) in CBF observed between acute and chronic time points. Similar findings of acute time point reductions in CBF (p=0.001) and CBV (p=0.002) were also seen in the core area. In contrast, CBF and CBV levels in the ICH core region appeared to worsen slightly at the sub-acute time point and then improve at the chronic time point. The degree of recovery in the core between acute and chronic time points, however, was not significant.
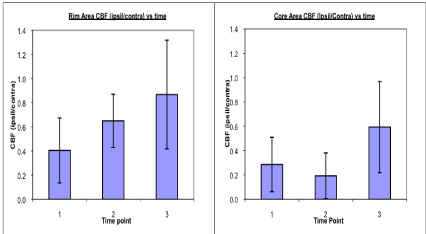
Figure 4: Average CBF ratio (ipsilateral/contralateral) values shown plotted as a function of time post-ICH. CBF ratios are shown for the adjacent rim (left) and the
central core (right) regions of interest at acute (1), sub-acute (2) and chronic (3) time points. CBF levels in both the rim and core areas were low acutely. CBF in the
rim, however, appeared to improve significantly over time, but not in the core.
Lastly, estimates of ADC are shown in Figure 5. The rim area demonstrated no significant changes at any of the time points (p>0.2) relative to contralateral values or across time; however, in the hematoma core region ADC values declined significantly (p=0.01) at the acute time before rebounding back to or slightly above contralateral levels at the subacute and chronic time points. A significant increase in core ADC values (p=0.05) was also noted between the acute and chronic studies. It should be noted that although DTI methods were used to measure ADC, we were not able to generate estimates of fractional anisotropy in the ICH region due to poor signal to noise.
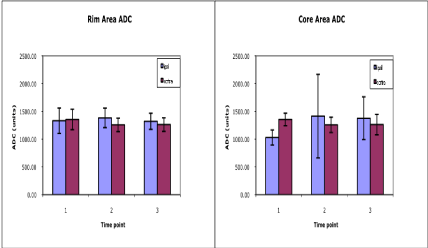
Figure 5: Average ADC values plotted as a function of time post-ICH. ADC values for the adjacent rim (left) and the central core (right) regions of interest located
in the ipsilateral and corresponding contralateral hemispheres are shown at acute (1), sub-acute (2) and chronic (3) time points. The initial decline of ADC in the
core area, while not reaching the same degree as seen with ischemic stroke, may be indicative of low CBF that eventually causes permanent injury. Conversely,
there were no significant ADC changes noted in the rim area.
Discussion
Using serial multiparametric MRI methods, this small, prospective safety trial of atorvastatin treatment following acute ICH demonstrated no hemorrhage recurrence in subjects with a GCS of >5. All subjects showed ICH resolution and lesion size decreased with time. It should be noted, however, that the loci of ICH in all subjects were deep with no lobar participation. While acute CBF and CBV levels suggest lower blood flow in the ICH rim, the ADC did not demonstrate corresponding changes that would be associated ischemia. Conversely, MRI measures from the core area showed sustained low CBF values concurrent with a small reduction in ADC levels acutely and significant elevation of ADC at the chronic time point suggesting irreversible injury in this region.
Patient #
Age
Sex
BP
Total Cholesterola
(Day 1)
Total Cholesterola (Day 7)
Total Cholesterola
(3 month)
GCS (admission)
GOS
(3 month)
Hematoma
Volume (cm3)
(acute)
Hematoma
Volume
(cm3)
(chronic)
1
39
M
242/134
153
125
203
15
4
32
2.6
2
42
F
148/108
197
144
230
6
5
44.2
4.3
3
52
F
175/101
208
171
199
15
5
9.4
1.0
4
52
F
179/112
151
98
128
15
--
18.1
--
5
57
M
189/111
139
89
131
15
4
58.4
10.3
6
73
M
211/162
105
92
--
13
4
16.1
1.8
a, mg/dl; --, data unavailable
Table 1: Summary of clinical data of patients included in the study.
The observations herein support previous reports on statin use before and/or after ICH [36] and decision analysis-based recommendations on possible statin use in acute non-lobar ICH [25]. Furthermore, imaging may be of assistance in the clinical approach to statin use for ICH. In the pharmacological treatment of clinical ICH, there is an opportunity to improve patient recovery, as most will not benefit significantly from current application of surgical clot removal, although additional minimally invasive, novel catheter based clot removal strategies such as Surgical Trial in Traumatic intra Cerebral Haemorrhage (STICH) have been investigated [37]. In addition, medical-based therapy with clot stabilization has not become a standard treatment [20,21,37,38]. The recently published STICH-II trial results, however, argue for surgical intervention in superficial lobar ICH without ventricular hemorrhage [39]. Thus, even while excluding drug therapy for purely lobar hemorrhages, a pharmacological approach may be of some benefit in non-lobar ICH.
Statin use in the context of a recent stroke, either ischemic or hemorrhagic, has undergone a surge of recent interest as the multifaceted beneficial effects of these compounds have been observed in the laboratory as well as in clinical studies. In the large Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial, high-dose atorvastatin (80 mg) administered after a transient ischemic attack or ischemic stroke was found to be clinically beneficial with an absolute 5-year stroke risk reduction of 2.2% [5,40]. In two independent clinical studies, prior statin use in patients who experienced an ICH was associated with reduced mortality and a higher chance of a good outcome [18,20,21]. Experimentally, statins have been shown to be neuroprotective and endothelial protective acutely after stroke [41]. Statins also improve brain recovery subacutely due to their influence on endogenous progenitor cells and by enhancing neurogenesis and angiogenesis [12,15].
In ICH patients that have undergone craniotomy with biopsy of the perihematomal regions, there is evidence of neurogenesis in the adult brain [42]. A medical therapy, such as a statin, which might increase this endogenous brain neurogenesis has therapeutic appeal because of the broad window of administration during the days and weeks after ICH. In laboratory studies using a rodent intracerebral blood injection model of ICH, statins improved neurological function by 2 weeks post ICH, and induced angiogenesis, neurogenesis, and synaptogenesis by 4 weeks after ICH [12,13]. Using the collagenase model of ICH in rats, atorvastatin-treated animals demonstrated significantly improved neurological functional scores and less hemispheric atrophy, as well as less brain edema at 3 days post ICH [11]. In more recent animal studies, we have used MRI to measure physiological and structural changes in the brain across time in statin-treated animals; there was good correlation between tissue loss measurements by MRI and histological methods, and both methods showed less tissue loss with atorvastatin treatment [12]. In addition, there was significant reduction of BBB permeability and edema, with increased CBF in the post ICH statin-treated animals [15]. These findings suggest the need for further statin treatment studies in clinical ICH patients.
Relationship between decreasing total and low density lipoprotein (LDL) levels and elevated risk of ICH has been suggested by some studies. For instance, Phuah and co-workers reported a steep decrease in total cholesterol and LDL levels 6 months prior to the onset of ICH in a patient population of 212 [43]. Most patients in this study were reported to have serum levels of total cholesterol between 170-180 mg/dl, a mean decrease from about 190 mg/dl 24 months pre-ICH. The authors suggested a causative link between accelerated decline in cholesterol and the risk of ICH. The total cholesterol levels in the population we studied ranged between 105-208 mg/ dl at ICH onset and exceeded the narrow range reported by these authors. More work is clearly needed to firmly establish any links between increased risk of ICH and rapid decline in total cholesterol and LDL levels. Possible mechanisms are suggested to be weakening of endothelial and microvascular walls due to increased broad inflammatory processes that are reflected in quick lipid reduction or direct structural weakening of vascular walls due to changes in membrane phospholipid composition [44]. In addition, Eisa-Beygi and Rezaei suggest that mutations in the genes or pharmacological perturbations of their gene-products and signaling mechanisms that regulate developmental neurovascular stabilization are likely to underlie the etiology of some forms of spontaneous ICH in humans [45]. However, despite this complex scenario, the clinical benefits of statin uses are believed to outweigh a slightly increased probability of post-stoke bleeding in the population at risk [46].
In the current study, atorvastatin was administered for 12 days after ICH; clinical features were noted and both early and delayed MRI studies were obtained. This represents the first prospective trial of a statin after ICH with correlative MRI analysis. These findings highlight the feasibility of combining a clinical therapeutic with advanced imaging to follow patient recovery. Yoshioka and colleagues employed MRI to follow outcome after ICH without experimental treatment, and have shown that fractional anisotropy using diffusion tensor tractography could predict motor functional outcome [47]. In the current study ICH induced changes in CBF, CBV, edema, ADC, and BBB permeability were measured. Edema increased in the rim acutely, as CBF was reduced, but both normalized subacutely. Acute ADC values in the rim were not significantly affected so there was no evidence for ischemia, consistent with previous laboratory [48,49] and clinical investigations [50,51]. Some drawbacks in the present study are the relatively small sample size and lack of variations in ICH location along with the absence of a placebo control group. Nonetheless, the results serve as a proof of the principle and warrant larger trials. This is strengthened by other similarities in the present results to previous reports. For instance, 12 month mortality rate was 50% in a non-statin ICH group compared to 33.7% in a statinexposed group [23]. In another study, statin use was the only variable also associated with lower 6-month mortality rate (12.5% in nonstatin vs 0% in statin cohort); this study also reported a GOS of 4-5 in 55% of statin-treated patients compared to 49.6% of controls [22], although no data on lesion size was reported. The present data also corroborate these data and also others from a previous report that showed no association between post-ICH statins and increased bleeding recurrence; for instance, Fitz Maurice, et al. have reported no increased rehemorrhage risk after post-ICH statin therapy [36].
Conclusion
Atorvastatin after ICH was safe and feasible in this study with no rebleeding observed following statin treatment. It should also be noted that none of the patients studied experienced any adverse effects during the study, and the planned MRI sequences were tolerable and obtainable. However, a potential weakness of this preliminary safety trial is non-inclusion of an untreated control group. Therefore, considering the prevailing paucity of effective treatments [52], future larger trials will require a comparison control group for MRI analysis to completely discern the statin treatment effects in acute ICH. There is also a need to address the known pleiotropic effects of the different statins available, their probable varying degrees of efficacies alone and in combination with other putative neurovascular unit rescuers, in appropriate preclinical models [7].
Acknowledgements
#These authors contributed equally to the manuscript. The authors thank all the study participants. Research reported in this publication was supported by the National Institute for Neurological Diseases and Stroke of the National Institutes of Health under award numbers R01NS058581 and P01NS23393. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- NINDS. IW. Priorities for clinical research in intracerebral hemorrhage: report from a National Institute of Neurological Disorders and Stroke workshop. Stroke. 2005; 36: e23-e41.
- Felberg RA, Grotta JC, Shirzadi AL, Strong R, Narayana P, Hill-Felberg SJ, et al. Cell death in experimental intracerebral hemorrhage: the "black hole" model of hemorrhagic damage. Ann Neurol. 2002; 51: 517-524.
- Morgenstern LB, Hemphill JC, Anderson C, Becker K, Broderick JP, Connolly ES, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010; 41: 2108-2129.
- Amin-Hanjani S, Stagliano NE, Yamada M, Huang PL, Liao JK, Moskowitz MA. Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke. 2001; 32: 980-986.
- Amarenco P, Bogousslavsky J, Callahan III A, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006; 355: 549-559.
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003; 53: 743-751.
- Zhang L, Zhang ZG, Chopp M. The neurovascular unit and combination treatment strategies for stroke. Trends Pharmacol Sci. 2012; 33: 415-422.
- Cui JJ, Wang D, Gao F, Li YR. Effects of atorvastatin on pathological changes in brain tissue and plasma MMP-9 in rats with intracerebral hemorrhage. Cell Biochem Biophys. 2012; 62: 87-90.
- Ewen T, Qiuting L, Chaogang T, Tao T, Jun W, Liming T, et al. Neuroprotective effect of atorvastatin involves suppression of TNF-a and upregulation of IL-10 in a rat model of intracerebral hemorrhage. Cell Biochem Biophys. 2013; 66: 337-346.
- Indraswari F, Wang H, Lei B, James ML, Kernagis D, Warner DS, et al. Statins improve outcome in murine models of intracranial hemorrhage and traumatic brain injury: a translational approach. J Neurotrauma. 2012; 29: 1388-1400.
- Jung KH, Chu K, Jeong SW, Han SY, Lee ST, Kim JY, et al. HMG-CoA reductase inhibitor, atorvastatin, promotes sensorimotor recovery, suppressing acute inflammatory reaction after experimental intracerebral hemorrhage. Stroke. 2004; 35: 1744-1779.
- Karki K, Knight RA, Han Y, Yang D, Zhang J, Ledbetter KA, et al. Simvastatin and atorvastatin improve neurological outcome after experimental intracerebral hemorrhage. Stroke. 2009; 40: 3384-3389.
- Seyfried DM, Han Y, Lu D, Chen J, Bydon A, Chopp M. Improvement in neurological outcome after administration of atorvastatin following experimental intracerebral hemorrhage in rats. J Neurosurg. 2004; 101: 104-107.
- Yang D, Knight RA, Han Y, Karki K, Zhang J, Chopp M, et al. Statins protect the blood brain barrier acutely after experimental intracerebral hemorrhage. J Behav Brain Sci. 2013; 3: 100-106.
- Yang D, Knight RA, Han Y, Karki K, Zhang J, Ding C, et al. Vascular recovery promoted by atorvastatin and simvastatin after experimental intracerebral hemorrhage: magnetic resonance imaging and histological study. J Neurosurg. 2011; 114: 1135-1142.
- Yang D, Knight RA, Han Y, Karki K, Zhang J, Ding C, et al. Atorvastatin and simvastatin promote vascular recovery after experimental intracerebral hemorrhage: MRI and histological study. J Neurosurg. 2011; 114: 1135-1142.
- Katsuki H. Exploring neuroprotective drug therapies for intracerebral hemorrhage. J Pharmacol Sci. 2010; 114: 366-378.
- Leker RR, Khoury ST, Rafaeli G, Shwartz R, Eichel R, Tanne D. Prior use of statins improves outcome in patients with intracerebral hemorrhage: prospective data from the National Acute Stroke Israeli Surveys (NASIS). Stroke. 2009; 40: 2581-2584.
- McGirt MJ, Pradilla G, Legnani FG, Thai QA, Recinos PF, Tamargo RJ, et al. Systemic administration of simvastatin after the onset of experimental subarachnoid hemorrhage attenuates cerebral vasospasm. Neurosurgery. 2006; 58: 945-951.
- Naval NS, Abdelhak TA, Urrunaga N, Zeballos P, Mirski MA, Carhuapoma JR. An association of prior statin use with decreased perihematomal edema. Neurocrit Care. 2008; 8: 13-18.
- Naval NS, Abdelhak TA, Zeballos P, Urrunaga N, Mirski MA, Carhuapoma JR. Prior statin use reduces mortality in intracerebral hemorrhage. Neurocrit Care. 2008; 8: 6-12.
- Tapia-Pérez JH, Rupa R, Zilke R, Gehring S, Voellger B, Schneider T. Continued statin therapy could improve the outcome after spontaneous intracerebral hemorrhage. Neurosurg Rev. 2013; 36: 279-287.
- Winkler J, Shoup JP, Czap A, Staff I, Fortunato G, Louise D. McCullough LD, et al. Long-term improvement in outcome after intracerebral hemorrhage in patients treated with statins. J Stroke Cerebrovasc Dis. 2013; 22: e541-e545.
- Goldstein LB. Statins after intracerebral hemorrhage: To treat or not to treat. Arch Neurol. 2011; 68: 565-566.
- Koch S. Intracerebral hemorrhage: Preventing recurrence of ICH - should statins be avoided? Nature Rev Neurol. 2011; 7: 193-194.
- Westover MB, Bianchi MT, Eckman MH, Steven M, Greenberg SM. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011; 68: 573-579.
- Bustamante A, Montaner J. Statin therapy should not be discontinued in patients with intracerebral hemorrhage. Stroke. 2013; 44: 2060-2061.
- Goldstein LB. Statin therapy should be discontinued in patients with intracerebral hemorrhage. Stroke. 2013; 44: 2058-2059.
- Tapia-Pérez JH, Sanchez-Aguilar M, Schneider T. The role of statins in neurosurgery. Neurosurg Rev. 2010; 33: 259-270.
- Tuch DS. Q-ball imaging. Magn Reson Med. 2004; 52: 1358-1372.
- Tuch DS, Reese TG, Wiegell MR, Wedeen VJ. Diffusion MRI of complex neural architecture. Neuron. 2003; 40: 885-895.
- Engvall C, Ryding E, Wirestam R, Holtås S, Ljunggren K, Ohlsson T, et al. Human cerebral blood volume (CBV) measured by dynamic susceptibility contrast MRI and 99mTc-RBC SPECT. J Neurosurg Anesthesiol. 2008; 20: 41-44.
- Ge Y, Law M, Johnson G, Herbert J, Babb JS, Mannon LJ, et al. Dynamic susceptibility contrast perfusion MR imaging of multiple sclerosis lesions: characterizing hemodynamic impairment and inflammatory activity. AJNR Am J Neuroradiol. 2005; 26: 1539-1547.
- Law M, Yang S, Babb JS, Knopp EA, Golfinos JG, Zagzag D, et al. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol. 2004; 25: 746-755.
- Welch KM, Nagesh V, D'Olhaberriague LD, Zhang ZG, Boska MD, Patel S, et al. Automated three-dimensional signature model for assessing brain injury in emergent stroke. Cerebrovasc Dis. 2001; 11: 9-14.
- FitzMaurice E, Wendell L, Snider R, Schwab K, Chanderraj R, Kinnecom C, et al. Effect of statins on intracerebral hemorrhage outcome and recurrence. Stroke. 2008; 39: 2151-2154.
- Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomized trial. Lancet. 2005; 365: 387-397.
- Barras CD, Tress BM, Christensen S, MacGregor L, Collins M, Desmond PM, et al. Density and shape as CT predictors of intracerebral hemorrhage growth. Stroke. 2009; 40: 1325-1331.
- Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013; 382: 397-408.
- Goldstein LB, Amarenco P, Zivin JA, Messig M, Altafullah I, Callahan A, et al. Statin treatment and stroke outcome in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2009; 40: 3526-3531.
- Nagaraja TN, Knight RA, Croxen RL, Konda KP, Fenstermacher JD. Acute neurovascular unit protection by simvastatin in transient cerebral ischemia. Neurol Res. 2006; 28: 826-830.
- Shen J, Xie L, Mao X, Zhou Y, Zhan R, Greenberg DA, et al. Neurogenesis after primary intracerebral hemorrhage in adult human brain. J Cereb Blood Flow Metab. 2008; 28: 1460-1468.
- Phuah CL, Raffeld MR, Ayres AM, Viswanathan A, Greenberg SM, Biffi A, et al. Subacute decline in serum lipids precedes the occurrence of primary intracerebral hemorrhage. Neurology. 2016; 86: 2034-2041.
- Mendelson SJ, Prabhakaran S. Lipid levels: A novel biomarker of impending intracerebral hemorrhage? Neurology. 2016; 86: 2028-2029.
- Eisa-Beygi S, Rezaei M. Progress into the etiology of intracerebral hemorrhage (ICH): Insights from Zebrafish embryos. Int J Dev Biol. 2016; 60: 119-126.
- Goldstein LB, Nederkoorn PJ. Statins and poststroke intracerebral hemorrhage: Concern but increasing reassurance. Neurology. 2016; 86: 1570-1571.
- Yoshioka H, Horikoshi T, Aoki S, Hori M, Ishigame K, Uchida M, et al. Diffusion tensor tractography predicts motor functional outcome in patients with spontaneous intracerebral hemorrhage. Neurosurgery. 2008; 62: 97-103.
- Orakcioglu B, Becker K, Sakowitz OW, Unterberg A, Schellinger PD. Serial diffusion and perfusion MRI analysis of the perihemorrhagic zone in a rat ICH model. Acta Neurochir Suppl. 2008; 103: 15-18.
- Orakcioglu B, Fiebach JB, Steiner T, Kollmar R, Jüttler E, Becker K, et al. Evolution of early perihemorrhagic changes - ischemia vs. edema: an MRI study in rats. Exp Neurol. 2005; 193: 369-376.
- Kang BK, Na DG, Ryoo JW, Byun HS, Roh HG, Pyeun YS. Diffusion-weighted MR imaging of intracerebral hemorrhage. Korean J Radiol. 2001; 2: 183-191.
- Prabhakaran S, Gupta R, Ouyang B, John S, Temes RE, Mohammad Y, et al. Acute brain infarcts after spontaneous intracerebral hemorrhage: a diffusion-weighted imaging study. Stroke. 2010; 41: 89-94.
- Xi G, Strahle J, Hua Y, Keep RF. Progress in translational research on intracerebral hemorrhage: Is there an end in sight? Progress Neurobiol. 2014; 115: 45-63.