Editorial
Austin J Clin Cardiolog. 2014;1(1): 1006.
What do we need to seek in the next step in Clinical Cardiology for Better Treatment of Patients with Cardiovascular Diseases?
Manabu Shirotani*, KiyonoriTogi and Noritsugu Uemori
Department of Cardiology, Nara Hospital, Kinki University Faculty of Medicine, Japan
*Corresponding author: : Manabu Shirotani, Department of Cardiology, Nara Hospital, Kinki University Faculty of Medicine, 1248-1, Otoda-Cho, Ikoma, Nara 630-0293, Japan
Received: January 23, 2014; Accepted: January 25, 2014; Published: January 27, 2014
With the marked development of catheterization techniques and medical instruments together with that of underlying supportive biological, physiological, pharmacological research, and metallurgical engineering, percutaneous vascular intervention has grown to be able to treat patients not only by dilatation with simple ballooning, but also by debulking atheromatous plaques or scaffolding stenotic lesions with metal stents with more ease [1]. Imaging devices provide more accurate assessment of lesion characteristics and severity than an angiogram, and often help interventionists decide how and how much they should dilate lesions. Stents have enabled much better procedural success in both coronary and peripheral artery disease, and excellent long-term patency has been achieved by using drugeluting stents (DES). However, there are still issues to be solved for better management of these patients, especially those with severely tight or calcified lesions, seriously depressed left ventricular (LV) function, or renal insufficiency.
In percutaneous coronary intervention (PCI), DES have been reported to reduce the restenosis rate, target lesion or vessel revascularization (TLR or TVR) rate, and thereby major adverse cardiac events (MACE), compared to uncoated bare metal stents (BMS) [2-5]. However, it is also reported that neoatherosclerosis, developing in the stented segment with new lipid-laden atherogenic change over the stent struts, is slowly created and limits blood flow and hence, the advantages over BMS years after the procedure [6- 8]. Is this true of second-generation DES, showing comparable or even better long-term outcomes with less frequent stent thrombosis (ST) than former DES [9-15], in which polymers, antiproliferative agents, and stent structure are believed to be improved? It is unknown whether such stenotic changes should also be expected with newer DES after a much longer interval as “very late catch–up”, because we have just started to obtain 5–year follow–up data [16].
In patients with acute myocardial infarction (AMI), emergency primary angioplasty to recanalize the occluded infarct–related artery is currently regarded as standard therapy for reducing cardiac mortality. In the balloon–only era, rapid closure of the infarcted lesion often took place after balloon dilatation [17]. Stent implantation, by blocking lesion recoil, proved to be quite beneficial to overcome this phenomenon, avoiding time–consuming repetitive balloon inflations until restoration of vessel patency. However, restenosis is still reported to be fairly frequent within a year with BMS use, especially at the time of implantation in the proximal left anterior descending artery compared with stenting in other coronary segments [18,19]. Currently, DES is used with more satisfactory TLR or TVR results during a 1– to 3–year follow–up period, although no significant difference is often observed between these two stent types in terms of the incidence of death or recurrent AMI [20–24]. However, accumulated data have begun to show a higher risk of ST in those treated with DES over a 1–year follow–up period [23–26]. Kalesan et al. [27] demonstrated in a meta–analysis that 1 year after AMI treatment, very late ST is significantly more likely to occur in patients receiving DES than in those with BMS placement. The large amount of intracoronary thrombus in patients with AMI may predispose them to stent malapposition because of stent undersizing or thrombus resolution. This may elevate the incidence of later ST, unless appropriate intimal coverage of the stent adluminal surface is achieved. This means that longer close management with steady antiplatelet treatment may be needed once patients with AMI are treated with DES. Longer followup studies of DES placement, particularly second–generation DES in AMI, are awaited.
As regards the treatment of unprotected left main coronary artery (LMCA) disease, PCI, in contrast to coronary bypass surgery (CABG), has been demonstrated to provide comparable long–term outcomes in terms of mortality and MACE, although a higher frequency of TVR must be expected [28–33]. However, ST in LMCA is highly likely to cause extremely dangerous hemodynamic deterioration, and even mortality, whereas occlusion of one graft conduit is not usually as harmful in patients undergoing CABG due to its smaller supply of the myocardial area than LMCA [34]. Therefore, considering the treatment of LMCA using DES, prevention of very late ST is of significant long–term importance. ST can occur unexpectedly a long time after stent implantation when insufficient attention is paid by both cardiologists and patients themselves. Once DES is implanted, even if ST is a low probability, cautious and close management and occasional follow–up examinations must be continued for a long period of time. Prolonged medication with antiplatelet agents may not be reduced as a result of symptomatic stability, which is obviously accompanied by more financial cost. When considered from the standpoint of patients, stenting to LMCA may involve extremely high mental stress from the fear of sudden death. Therefore, PCI and CABG in an unprotected LMCA lesion should be compared because of the considerably long follow–up period, not only from the view of the clinical outcome but also from the viewpoint of patients’ mental strain and financial costs associated with clinical care, which must be secured in patients receiving DES.
Other new PCI devices are becoming available. Treatment of both de novo lesions and in–stent restenosis by drug–eluting balloons can be useful [35–37] as it does not add more metal foreign bodies to the lesion that could promote further inflammatory reactions or clot formation. Bioresorbable vessel scaffolding (BVS) may also be substituted for stents and possibly requires a shorter period of antiplatelet therapy [38,39]. It is of interest how BVS acts at a bifurcating lesion when it is positioned over the side branch. Development of these new devices may also change the utilization of modalities assisting in the judgment of the indication, lesion characteristics, and procedural endpoint among intravascular ultrasound, optical coherence tomography, or a pressure guidewire to measure fractional flow reserve.
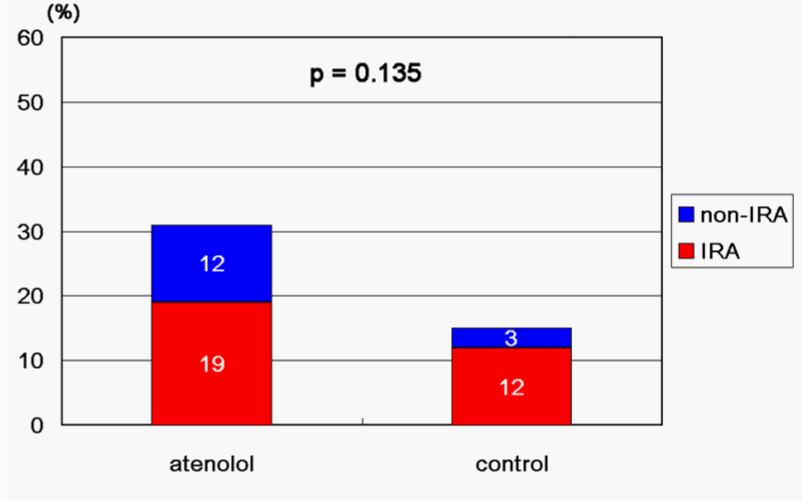
Subsequent to successful coronary recanalization in AMI patients, are beta–blockers additionally able to improve the long–term clinical course? This question remains to be further elucidated [40,41]. Beta–blockers are known to inhibit adverse arrhythmia [42] and are also known to improve LV contractility [43–45]. However, there are concerns about inducing coronary vasoconstriction as a result of their beta–blocking effect on coronary vascular smooth muscle cells (VSMC) [46,47]. Coronary vasospasm is reported to be commonly induced early after AMI onset. Racial differences in its frequency were demonstrated by Pristipino et al. [48], in which Japanese patients showed more provocative coronary vasospasm (80%) than Caucasians (still as high as 37% within 14 days of AMI onset). It was reported in a study of a small number of Japanese subjects that atenolol tended to increase provocative coronary vasospasm in both the infarct–related artery (IRA) and non–IRA, although it did not reach statistical significance (Figure 1) [49]. With reference to beta–blockers, their various cardiac effects should be taken into consideration. Moreover, their actions can vary depending upon the pharmacological characteristics (e. g. beta1–adrenoceptor selectivity, intrinsic sympathomimetic activity) of each beta–blocking agent used. Their short– and long–term influence on AMI patients should be evaluated in large–scale randomized trials, paying attention to the agent (s) used and the racial background of subjects.
Coronary vasospastic angina has been diagnosed by the spasm provocation test during coronary angiography. Intracoronary administration of acetylcholine (Ach) and ergonovine is commonly used. However, the pattern of provoked spasm is reported to be different between the two agents; more diffuse with the former and more focal with the latter [50]. Patients can show positive in a test performed using one agent, yet sometimes pseudo–negative in a test using another [50,51], although usually only one is used in the daily clinical setting. Back–up temporary pacing is necessary for the Ach provocation test because it frequently causes heart block. This test is sometimes complicated with dangerous ventricular arrhythmia together with ST elevation on the electrocardiogram. Considering the financial cost and safety, a less invasive diagnostic method is expected to be explored. A couple of reports have suggested the association with the elevation of plasma inflammatory or oxidative stress biomarkers [52–55]; however, they can also be influenced if other pathological conditions such as infection or collagen disease coexist. Sakata et al. [56] suggested the efficacy of assessing regional myocardial autonomic nerve activity by means of nuclear medicine imaging. The leukocyte Rho–kinase level has been shown to be elevated in patients with coronary vasospasm [57,58]. Rho/Rho–kinase has been demonstrated to play a pivotal role in promoting VSMC contraction by enhancing myosin light chain phosphorylation via suppression of the myosin–binding unit of myosin phosphatase [59,60]. Further studies are awaited to develop easier and more efficacious diagnosis of this disease and its variable vasomotor activities.
Figure 1: Frequency of inducible coronary vasospasm by ergonovine 4 weeks after AMI onset. IRA: infarct-related artery.
Cell therapy is now becoming an attractive field in the treatment of chronic heart failure or restoration of LV function after AMI. Many attempts using a variety of cell origins (e. g. autologous bone marrow cells, peripheral blood stem cells) have been reported; however, the long–term beneficial effects remain controversial, including concerns about promoting intimal hyperplasia following coronary intervention [61–71]. New technologies such as surgical coverage of the damaged LV with a myocardial sheet produced by autologous cell culture or transformation of induced pluripotent stem cells [72,73] may emerge as promising therapeutic options. Together with basic translational research, rapid but solid development in this field is awaited with great hope and expectation.
A number of other important issues remain to be solved in addition to the above issues. We hope that many exciting studies will be submitted to this journal to develop fresh diagnostic and therapeutic methods. Such advancements will surely contribute to more efficient clinical practice to refine the management of cardiovascular diseases.
References
- Lemos PA, Farooq V, Takimura CK, Gutierrez PS, Virmani R, Kolodgie F, et al. Emerging technologies: polymer-free phospholipid encapsulated sirolimus nanocarriers for the controlled release of drug from a stent-plus-balloon or a stand-alone balloon catheter. EuroIntervention. 2013; 9: 148-156.
- Applegate RJ, Sacrinty MT, Kutcher MA, Santos RM, Gandhi SK, Little WC. Effect of length and diameter of drug-eluting stents versus bare-metal stents on late outcomes. Circ Cardiovasc Interv. 2009; 2: 35-42.
- Bangalore S, Kumar S, Fusaro M, Amoroso N, Attubato MJ, Feit F, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012; 125: 2873-2891.
- Applegate RJ, Sacrinty MT, Kutcher MA, Santos RM, Gandhi SK. Little WC.3-year comparison of drug-eluting versus bare-metal stents. JACC CardiovascInterv. 2009; 2: 231-239.
- Tsai TT, Messenger JC, Brennan JM, Patel UD, Dai D, Piana RN, et al. Safety and eficacy of drug-eluting stents in older patients with chronic kidney disease: a report from the linked CathPCI Registry-CMS claims database. J Am Coll Cardiol. 2011; 58: 1859-1869.
- Kang SJ, Mintz GS, Akasaka T, Park DW, Lee JY, Kim WJ, et al. Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug- eluting stent implantation. Circulation. 2011; 123: 2954-2963.
- Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011; 57: 1314-1322.
- Yonetsu T, Kato K, Kim SJ, Xing L, Jia H, McNulty I, Lee H, Zhang S, Uemura S, Jang Y, Kang SJ, Park SJ, Lee S, Yu B, Kakuta T, Jang IK. Predictors for neoatherosclerosis: a retrospective observational study from the optical coherence tomography registry. CircCardiovasc Imaging. 2012; 5: 660-666.
- Kedhi E, Gomes ME, Lagerqvist B, Smith JG, Omerovic E, James S, et al. Clinical impact of second-generation everolimus-eluting stent compared with irst-generation drug-eluting stents in diabetes mellitus patients: insights from a nationwide coronary intervention register. JACC CardiovascInterv. 2012; 5: 1141-1149.
- Simsek C, Räber L, Magro M, Boersma E, Onuma Y, Stefanini GG, et al. Long-term outcome of the unrestricted use of everolimus-eluting stents compared to sirolimus-eluting stents and paclitaxel-eluting stents in diabetic patients: the Bern-Rotterdam diabetes cohort study. Int J Cardiol. 2013; 170: 36-42.
- Jensen LO, Thayssen P, Hansen HS, Christiansen EH, Tilsted HH, Krusell LR, et al. Scandinavian Organization for Randomized Trials With Clinical Outcome IV (SORT OUT IV) Investigators.Randomized comparison of everolimus-eluting and sirolimus-eluting stents in patients treated with percutaneous coronary intervention: the Scandinavian Organization for Randomized Trials with Clinical Outcome IV (SORT OUT IV). Circulation. 2012; 125: 1246-1255.
- Räber L, Jüni P, Nüesch E, Kalesan B, Wenaweser P, Moschovitis A, et al. Long-term comparison of everolimus-eluting and sirolimus-eluting stents for coronary revascularization. J Am Coll Cardiol. 2011; 57: 2143-2151.
- Danzi GB, Chevalier B, Urban P, Fath-Ordoubadi F, Carrie D, Wiemer M, et al. NOBORI 2 Investigators. Clinical performance of a drug-eluting stent with a biodegradable polymer in an unselected patient population: the NOBORI 2 study. EuroIntervention. 2012; 8: 109-116.
- Klauss V, Serruys PW, Pilgrim T, Buszman P, Linke A, Ischinger T, et al. 2-year clinical follow-up from the randomized comparison of biolimus-eluting stents with biodegradable polymer and sirolimus-eluting stents with durable polymer in routine clinical practice. JACC CardiovascInterv. 2011; 4: 887- 895.
- Tada T1, Byrne RA1, Simunovic I1, King LA1, Cassese S1, Joner M1, et al. Risk of stent thrombosis among bare-metal stents, irst-generation drug- eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv. 2013; 6: 1267-1274.
- Gada H, Kirtane AJ, Newman W, Sanz M, Hermiller JB, Mahaffey KW, Cutlip DE, Sudhir K, Hou L, Koo K, Stone GW. 5-Year Results of a Randomized Comparison of XIENCE V Everolimus-Eluting and TAXUS Paclitaxel- Eluting Stents: Final Results From the SPIRIT III Trial (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions). JACC CardiovascInterv. 2013; 6: 1263-1266.
- Shirotani M, Yui Y, Hattori R, Morishita H, Kawai C, Susawa T, et al. Emergency coronary angioplasty for acute myocardial infarction: predictors of early occlusion of the infarct-related artery after balloon inlation. Am Heart J. 1993; 125: 931-938.
- Shugman IM, Hee L, Mussap CJ, Diu P, Lo S, Hopkins AP, et al. Bare-metal stenting of large coronary arteries in ST-elevation myocardial infarction is associated with low rates of target vessel revascularization. Am Heart J. 2013; 165: 591-599.
- Calais F, Lagerqvist B, Leppert J, James SK, Fröbert O. Proximal coronary artery intervention: stent thrombosis, restenosis and death. Int J Cardiol. 2013; 170: 227-232.
- Spaulding C, Henry P, Teiger E, Beatt K, Bramucci E, Carriè D, et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med. 2006; 355: 1093-1104.
- Sabate M, Cequier A, Iñiguez A, Serra A, Hernandez-Antolin R, Mainar V, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012; 380: 1482-1490.
- Garro N, Capodanno D, Cammalleri V, Tamburino C. Very late thrombosis in acute myocardial infarction: drug-eluting versus uncoated stents. EuroIntervention. 2008; 4: 324-330.
- Assali A, Vaduganathan M, Vaknin-Assa H, Lev EI, Brosh D, Teplitsky I, et al. Comparison of late (3-year) registry data outcomes using bare metal versus drug-eluting stents for treating ST-segment elevation acute myocardial infarctions. Am J Cardiol. 2012; 109: 1563-1568.
- De Luca G, Dirksen MT, Spaulding C, Kelbaek H, Schalij M, Thuesen L, et al. Drug-Eluting Stent in Primary Angioplasty (DESERT) Cooperation.Drug- eluting vs bare-metal stents in primary angioplasty: a pooled patient-level meta-analysis of randomized trials. Arch Intern Med. 2012; 172: 611-621.
- Brodie B, Pokharel Y, Garg A, Kissling G, Hansen C, Milks S, et al. Predictors of early, late, and very late stent thrombosis after primary percutaneous coronary intervention with bare-metal and drug-eluting stents for ST-segment elevation myocardial infarction. JACC CardiovascInterv. 2012; 5: 1043-1051.
- Kukreja N, Onuma Y, Garcia-Garcia HM, Daemen J, van Domburg R, Serruys PW; Interventional Cardiologists of the Thoraxcenter (2000 to 2005). The risk of stent thrombosis in patients with acute coronary syndromes treated with bare-metal and drug-eluting stents. JACC Cardiovasc Interv. 2009; 2: 534- 541.
- Kalesan B, Pilgrim T, Heinimann K, Räber L, Stefanini GG, Valgimigli M, et al. Comparison of drug-eluting stents with bare metal stents in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012; 33: 977-987.
- Chieffo A, Meliga E, Latib A, Park SJ, Onuma Y, Capranzano P, et al. Drug- eluting stent for left main coronary artery disease. The DELTA registry: Multicenter registries evaluating percutaneous coronary intervention versus coronary artery bypass grafting for left main treatment. JACC CardiovascInterv. 2012; 5: 718-727.
- Kang SH, Park KH, Choi DJ, Park KW, Chung WY, Lim C, et al. Coronary artery bypass grafting versus drug-eluting stent implantation for left main coronary artery disease (from a two-center registry). Am J Cardiol. 2010; 105: 343-351.
- Palmerini T, Barlocco F, Santarelli A, Bacchi-Reggiani L, Savini C, Baldini E, Alessi L, Rufini M, Di Credico G, Piovaccari G, Di Bartolomeo R, Marzocchi A, Branzi A, De Servi S. A comparison between coronary artery bypass grafting surgery and drug eluting stent for the treatment of unprotected left main coronary artery disease in elderly patients (aged > or =75 years). Eur Heart J. 2007; 28: 2714-2719.
- Park DW, Kim YH, Yun SC, Lee JY, Kim WJ, Kang SJ, et al. Long-term outcomes after stenting versus coronary artery bypass grafting for unprotected left main coronary artery disease: 10-year results of bare-metal stents and 5-year results of drug-eluting stents from the ASAN-MAIN (ASAN Medical Center-Left MAIN Revascularization) Registry. J Am Coll Cardiol. 2010; 56: 1366-1375.
- Jiang WB, Zhao W, Huang H, Li CL, Zhang JH, Wang Y, et al. Meta-analysis of effectiveness of irst-generation drug-eluting stents versus coronary artery bypass grafting for unprotected left main coronary disease. Am J Cardiol. 2012; 110: 1764-1772.
- Athappan G1, Patvardhan E2, Tuzcu ME3, Ellis S3, Whitlow P3, Kapadia SR4. Left main coronary artery stenosis: a meta-analysis of drug-eluting stents versus coronary artery bypass grafting. JACC Cardiovasc Interv. 2013; 6: 1219-1230.
- Farooq V, Serruys PW, Zhang Y, Mack M, Ståhle E, Holmes DR, et al. Short- Term and Long-Term Clinical Impact of Stent Thrombosis and Graft Occlusion in the SYNTAX Trial at 5 Years: Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery Trial. J Am CollCardiol. 2013; 62: 2360-2369.
- Stella PR, Belkacemi A, Waksman R, Stahnke S, Torguson R, von Strandmann RP, Agostoni P, et al. The Valentines Trial: results of the irst one week worldwide multicentre enrolment trial, evaluating the real world usage of the second generation DIOR paclitaxel drug-eluting balloon for in-stent restenosis treatment. EuroIntervention. 2011; 7: 705-710.
- Wöhrle J, Zadura M, Möbius-Winkler S, Leschke M, Opitz C, Ahmed W, et al. SeQuentPlease World Wide Registry: clinical results of SeQuent please paclitaxel-coated balloon angioplasty in a large-scale, prospective registry study. J Am CollCardiol. 2012; 60: 1733-1738.
- Latib A, Colombo A, Castriota F, Micari A, Cremonesi A, De Felice F, et al. A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: the BELLO (Balloon Elution and Late Loss Optimization) study. J Am CollCardiol. 2012; 60: 2473- 2480.
- Ormiston JA, Serruys PW, Onuma Y, van Geuns RJ, de Bruyne B, Dudek D, et al. First serial assessment at 6 months and 2 years of the second generation of absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study. CircCardiovascInterv. 2012; 5: 620-632.
- Muramatsu T, Onuma Y, García-García HM, Farooq V, Bourantas CV, Morel MA, et al. Incidence and short-term clinical outcomes of small side branch occlusion after implantation of an everolimus-eluting bioresorbable vascular scaffold: an interim report of 435 patients in the ABSORB-EXTEND single- arm trial in comparison with an everolimus-eluting metallic stent in the SPIRIT irst and II trials. JACC CardiovascInterv. 2013; 6: 247-257.
- Ozasa N, Morimoto T, Bao B, Furukawa Y, Nakagawa Y, Kadota K, et al. β-blocker use in patients after percutaneous coronary interventions: one size its all? Worse outcomes in patients without myocardial infarction or heart failure. Int J Cardiol. 2013; 168: 774-779.
- Rochon PA, Tu JV, Anderson GM, Gurwitz JH, Clark JP, Lau P, et al. Rate of heart failure and 1-year survival for older people receiving low-dose beta- blocker therapy after myocardial infarction. Lancet. 2000; 356: 639-644.
- Valle JA, Zhang M, Dixon S, Aronow HD, Share D, Naoum JB, Gurm HS. Impact of pre-procedural beta blockade on inpatient mortality in patients undergoing primary percutaneous coronary intervention for ST elevation myocardial infarction. Am J Cardiol. 2013; 111: 1714-17120.
- Basu S, Senior R, Raval U, van der Does R, Bruckner T, Lahiri A. Beneicial effects of intravenous and oral carvedilol treatment in acute myocardial infarction. A placebo-controlled, randomized trial. Circulation. 1997; 96: 183- 191.
- Senior R, Basu S, Kinsey C, Schaeffer S, Lahiri A. Carvedilol prevents remodeling in patients with left ventricular dysfunction after acute myocardial infarction. Am Heart J. 1999; 137: 646-652.
- Poulsen SH, Jensen SE, Egstrup K. Effects of long-term adrenergic beta- blockade on left ventricular diastolic illing in patients with acute myocardial infarction. Am Heart J. 1999; 138: 710-720.
- Robertson RM, Wood AJ, Vaughn WK, Robertson D. Exacerbation of vasotonic angina pectoris by propranolol. Circulation. 1982; 65: 281-285.
- Tilmant PY, Lablanche JM, Thieuleux FA, Dupuis BA, Bertrand ME. Detrimental effect of propranolol in patients with coronary arterial spasm countered by combination with diltiazem. Am J Cardiol. 1983; 52: 230-233.
- Pristipino C, Beltrame JF, Finocchiaro ML, Hattori R, Fujita M, Mongiardo R, Cianlone D, Sanna T, Sasayama S, Maseri A. Major racial differences in coronary constrictor response between japanese and caucasians with recent myocardial infarction. Circulation. 2000; 101: 1102-1108.
- Shirotani M, Yokota R, Kouchi I, Hirai T, Uemori N, Haba K, et al. Inluence of atenolol on coronary artery spasm after acute myocardial infarction in a Japanese population. Int J Cardiol. 2010; 139: 181-186.
- Sueda S, Kohno H, Fukuda H, Ochi N, Kawada H, Hayashi Y, et al. Induction of coronary artery spasm by two pharmacological agents: comparison between intracoronary injection of acetylcholine and ergonovine. Coron Artery Dis. 2003; 14: 451-457.
- Suzuki Y, Tokunaga S, Ikeguchi S, Miki S, Iwase T, Tomita T, et al. Induction of coronary artery spasm by intracoronary acetylcholine: comparison with intracoronary ergonovine. Am Heart J. 1992; 124: 39-47.
- Li JJ, Zhang YP, Yang P, Zeng HS, Qian XW, Zhang CY, et al. Increased peripheral circulating inlammatory cells and plasma inlammatory markers in patients with variant angina. Coron Artery Dis. 2008; 19: 293-297.
- Hung MJ, Cherng WJ, Yang NI, Cheng CW, Li LF. Relation of high-sensitivity C-reactive protein level with coronary vasospastic angina pectoris in patients without hemodynamically signiicant coronary artery disease. Am J Cardiol. 2005; 96: 1484-1490.
- Hung MJ, Cherng WJ, Cheng CW, Li LF. Comparison of serum levels of inlammatory markers in patients with coronary vasospasm without signiicant ixed coronary artery disease versus patients with stable angina pectoris and acute coronary syndromes with signiicant ixed coronary artery disease. Am J Cardiol. 2006; 97: 1429-1434.
- Yamashita K, Takahiro K, Kamezaki F, Adachi T, Tasaki H. Decreased plasma extracellular superoxide dismutase level in patients with vasospastic angina. Atherosclerosis. 2007; 191: 147-152.
- Sakata K, Miura F, Sugino H, Saegusa T, Shirotani M, Yoshida H, et al. Assessment of regional sympathetic nerve activity in vasospastic angina: analysis of iodine 123-labeled metaiodobenzylguanidine scintigraphy. Am Heart J. 1997; 133: 484-489.
- Hung MJ, Cherng WJ, Hung MY, Kuo LT, Cheng CW, Wang CH, et al. Increased leukocyte Rho-associated coiled-coil containing protein kinase activity predicts the presence and severity of coronary vasospastic angina. Atherosclerosis. 2012; 221: 521-526.
- Kikuchi Y, Yasuda S, Aizawa K, Tsuburaya R, Ito Y, Takeda M, et al. Enhanced Rho-kinase activity in circulating neutrophils of patients with vasospastic angina: a possible biomarker for diagnosis and disease activity assessment. J Am Coll Cardiol. 2011; 58: 1231-1237.
- Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005; 25: 1767- 1775.
- Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol. 2007; 50: 17-24.
- Tendera M, Wojakowski W, Ruzyllo W, Chojnowska L, Kepka C, Tracz W, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009; 30: 1313-1321.
- Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, at al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006; 113: 1287-1294.
- Yousef M, Schannwell CM, Köstering M, Zeus T, Brehm M, Strauer BE. The BALANCE Study: clinical beneit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. J Am Coll Cardiol. 2009; 53: 2262-2269.
- Meyer GP, Wollert KC, Lotz J, Pirr J, Rager U, Lippolt P, et al. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur Heart J. 2009; 30: 2978-2984.
- Kandala J, Upadhyay GA, Pokushalov E, Wu S, Drachman DE, Singh JP. Meta-analysis of stem cell therapy in chronic ischemic cardiomyopathy. Am J Cardiol. 2013; 112: 217-225.
- Huikuri HV, Kervinen K, Niemelä M, Ylitalo K, Säily M, Koistinen P, et al. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk proile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart J. 2008; 29: 2723-2732.
- Sürder D, Manka R, Lo Cicero V, Moccetti T, Ruibach K, Soncin S, Turchetto L, at al. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation. 2013;127: 1968-1979.
- Cao F, Sun D, Li C, Narsinh K, Zhao L, Li X, et al. Long-term myocardial functional improvement after autologous bone marrow mononuclear cells transplantation in patients with ST-segment elevation myocardial infarction: 4 years follow-up. Eur Heart J. 2009; 30: 1986-1994.
- Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011; 378: 1847-1857.
- Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004; 363: 751-756.
- Kang HJ, Kim MK, Lee HY, Park KW, Lee W, Cho YS, et al. Five-year results of intracoronary infusion of the mobilized peripheral blood stem cells by granulocyte colony-stimulating factor in patients with myocardial infarction. Eur Heart J. 2012; 33: 3062-3069.
- Miki K, Uenaka H, Saito A, Miyagawa S, Sakaguchi T, Higuchi T, et al. Bioengineered Myocardium Derived from Induced Pluripotent Stem Cells Improves Cardiac Function and Attenuates Cardiac Remodeling Following Chronic Myocardial Infarction in Rats. Stem Cells Transl Med. 2012; 1: 430- 437.
- Sawa Y, Miyagawa S. Present and Future Perspectives on Cell Sheet-Based Myocardial Regeneration Therapy. Biomed Res Int. 2013; 2013: 583912.