
Research Article
Austin J Dermatolog. 2015; 2(2): 1038.
Skin Capacitance Mapping of Early Effects of Methotrexate and Etanercept Biotherapy on Plaque Type Psoriasis
Gérald E Piérard1*, Sébastien L Piérard2 and Claudine Piérard- Franchimont3,4
1Laboratory of Skin Bioengineering and Imaging (LABIC), Department of Clinical Sciences, University of Liege, Belgium
2Telecommunications and Imaging, Laboratory INTELSIG, Montefiore Institute, Liege University, Belgium
3Department of Dermatopathology, Unilab Lg, University Hospital of Liege, Belgium
4Department of Dermatology, Regional Hospital of Huy, Belgium
*Corresponding author: Gérald E Piérard, Department of Clinical Sciences, University of Liege, Belgium
Received: July 30, 2015; Accepted: November 10, 2015; Published: November 12, 2015
Abstract
In its moderate-to-severe forms, psoriasis of the skin is an immune-mediated disorder that is commonly treated by biologicals. There is a need to design some objective noninvasive analytical methods for assessing the early signs of efficacy of such drugs. In recent years, in vivo real-time Skin Capacitance Mapping (SCM) has been offered for such evaluations. We presently assess SCM in two groups of 11 adult psoriatic patients treated with either Methotrexate (MTX) or Etanercept (ETC) monotherapies. Cyanoacrylate Skin Surface Strippings (CSSS) were collected at inclusion and after a 6-week period of treatment. The acute progression of psoriasis was identified by darker areas at SCM corresponding to subtle serosity deposits in the stratum corneum. CSSS and SCM seem to represent two noninvasive objective methods assessing the initial phase of psoriasis improvement following systemic therapies.
Keywords: Psoriasis; Skin capacitance mapping; Biologicals; Methotrexate; Etanercept; Cyanoacrylate skin surface stripping
Background
Psoriasis is a chronic disabling Immune-Mediated Inflammatory Disorder (IMID) of the skin commonly associated with an increased incidence of some comorbidity including arthropathies, cardiovascular disease, metabolic syndrome, obesity, and type 2 diabetes [1-3]. In moderate-to-severe skin psoriatic lesion grades, a mindful holistic approach to the patient, commonly relies on selective systemic anti-inflammatory treatments [1,4,5]. Some recent progress in skin immunopathology has upgraded the perception of psoriasis pathogenesis [6,7]. It fostered new treatment options based on rational advances for psoriatic patients [2]. When drugs are administered as monotherapies or combined treatments, proinflammatory cytokines, including Tumor Necrosis Factor (TNF) and specific interleukins (IL12 and 23) are possibly targeted. They globally exert a favourable balance between efficacy and safety [8,9].
The Psoriasis Area and Severity Index (PASI) score is commonly used at the drug selection at inclusion [10-14]. A severe PASI score is commonly a benchmark for selecting a monotherapeutic option including Methotrexate (MTX) and Etanercept (ETC) administrations. Unfortunately, there is a poor objective and uniform clinical selection among psoriasis patients in controlled trials, there are some safety concerns, and the assessment of the treatment efficacy remains limited on clinical ground [15].
Only a few noninvasive clinical methods are currently designed for assessing the initial treatment efficacy of plaque type psoriasis on an individual basis. One way compares the treatment duration leading to the reduction in PASI score by 75% (PASI 75). Similar comparisons are performed using PASI 50 and PASI 90 reductions. A distinct comparative procedure relies of in vivo real-time Skin Capacitance Mapping (SCM) that represents an innovative method in the field [3,16,17]. SCM records some electrometric properties of the skin that are influenced by the combination of Stratum Corneum (SC) texture and micro relief, its water content, its electrolyte components, and the sweat production [3,16-18]. The SCM method is rooted on water permittivity, which is higher than for most bio-molecular compounds. Still another procedure involves Cyanoacrylate Skin Surface Strippings (CSSS) that are conveniently collected from the margins of the psoriatic plaques (Figure 1). The method was previously described in details [19-21]. Psoriasis is fostered by peculiar activation of TH1 lymphocytes [6,7] and CD123+ plasmacytoid dendritic cells [22]. These cells release various pro-inflammatory cytokines [23]. The resulting complex process probably correlates positively with the severity of clinical presentations.
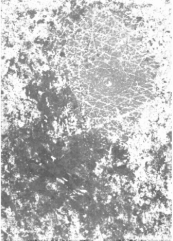
Figure 1: Heterogeneous pattern of SCM before systemic treatment of a
psoriasis plaque. High capacitance (dark area) corresponds to the acute IMID
phase (area : 8 x12.8 mm).
The aim of the present study was to compare the effects of intramuscular MTX and subcutaneous ETC over a 6-week period using the noninvasive SCM method and a minimally invasive collection of CSSS from the periphery of plaque type psoriasis. MTX is a systemic therapy that has demonstrated clinical efficacy in patients with psoriasis [24-27]. ETC is a fully human, soluble Tumour Necrosis Factor (TNF)-receptor fusion protein that is approved in the treatment of moderate to severe plaque psoriasis at a dosage of 50 mg twice weekly for up to 3 months, followed by 50 mg once weekly [5,28-33].
Patients and Method
The present observational study was approved by the Ethic Committee of the University Hospital of Liege. It was performed in accordance with the Declaration of Helskinki. The psoriatic patients were involved in a procedure of personalized medicine for the past 8 years. The trial was conducted with the understanding and consent of the volunteers. All patients provided written informed consent before performance of any study-related procedures. They were treated following the National Health regulations in Belgium limiting the prescription of expensive biological therapies. In the present study, noninvasive observations were performed in two groups of 11 adult patients suffering from progressive moderate-to-severe plaque type psoriasis. Their median age was 47 years (range: 38-61), and their body mass index ranged 19.3 - 23.6. They previously received PUVAtherapy in a routine procedure. At inclusion in the present therapeutic procedures, the outpatients were informed about the nature and risks associated with the treatments and about the aims and procedures of CSSS harvesting and SCM imagen captures. They received either intramuscular MTX (Pfizer) at a 15 mg/week dosage or subcutaneous ETC (Pfizer) at a 25 mg dosage twice a week.
Psoriatic lesions corresponded to stable plaques that were uncontrolled by the previous PUVA therapy. The patients were out of topical and systemic treatments for at least one-month prior entering the observational study phase. Eligible patients were aged over 18 years. Their global PASI score was above 13. They were not immunocompromised or taking drugs at risk of psoriasis exacerbation (lithium, beta- adrenergic blocker agents, anti-malarials,…). At entry in the observational phase (T0) and 6 weeks after initiating the MTX or ETC treatment (T6), the PASI score was calculated, and both CSSS and SCM aspects were recorded.
CSSS were examined under the microscope looking for serous and neutrophil collections with parakeratotic cells in the stratum corneum. In the present study, the SkinChip® device (L’Oreal, Paris, France) and the Moisture Map MM100 (CK technology, Vise, Belgium) were used for SCM. In each device, a set of microcapacitors were placed at 50 μm intervals on a 18 x 12.8 mm sensor probe (Figure 2). The devices were closely applied to the skin surface for about 5 seconds in order to avoid any interference with both the water evaporation and storage inside the SC [3,17,18]. Real time SCM measurements were transposed in a range of 256 gray levels of pixels to form non-optical SCM images. In psoriatic plaques, a large range of skin surface capacitance patterns were expected [16]. The typical SCM aspect of psoriasis plaques corresponds to a patchwork of darker and lighter spots. The darker pixels corresponded to hydrated high capacitance spots. By contrast, clear pixels were identified as dry spots of the skin, as well as creases in the SC micro relief, preventing close contact between the probe and the skin.

Figure 2: Low diffuse skin capacitance after a 6-week treatment with ETC
(area : 8 x12.8 mm).
In each volunteer, the relative area (%) of the darker spots was averaged for 5 contiguous fields. Results were expressed as Medians (Me) and ranges. The median areas at T0 and T6 were compared using the Mann-Whitney U test. Results were considered to be statistically significant at the 5% critical level (p<0.05). All calculations were performed using SAS (version 8.2 for Windows) and S-PLUS (version 6.1) statistical packages.
Results
Globally, the median PASI score was decreased from T0 to T6 for both drugs (Table 1). Adverse events were not observed with any of the treatments.
MTX
ETC
Parameters
Y0
P
T6
T0
P
T6
Number
11
11
11
11
Age (years, Me)
46
49
BMI (Me)
21.9
22.4
PASI (Me)
18
< 0.05
14.5
21
< 0.01
13
CSSS (pk, % area)
13.4
< 0.05
8.9
16.9
< 0.05
10.7
SCM (% serous spots, Me)
44.9
NS
48.3
67.2
< 0.01
40.1
Table 1: Evolution of psoriasis treated by Methotrexate (MTX) or Etanercept (ETC) between inclusion (T0) and a 6-week treatment (T6), Median (MC), Parakeratosis (pk), not significant charge (NS).
CSSS showed almost similar trends of changes in the psoriasis aspect for both treatment groups at T0 and T6 (Table 1). The extent in parakeratotic cells was significantly reduced at T6. Similar trends were found with spongiosis is CSSS. The density of clusters of neutrophils followed the same trend in reduction over time.
The SCM data were differently affected by the treatment modalities. In the MTX patients the relative area of the darker (serous) spots remained unchanged between T0 and T6. By contrast, in the ETC patients, the relative area of the darker spots decreased significantly in time (Table 1).
Discussion
MTX and ETC are well-established treatment modalities for moderate-to- severe presentations of psoriasis. MTX is highly effective for chronic plaque psoriasis, particularly in its severe forms. The drug was found to be effective at much lower doses than those required for curbing epidermal hyperproliferation. The anti- inflammatory action of MTX indeed results from the inhibition of an enzyme involved in purine metabolism. This leads to the accumulation of extracellular adenosine which displays potent anti-inflammatory activities, particularly for neutrophils. The therapeutic effects of MTX usually require 4-8 weeks to become evident [4,24,28].
ETC is a dimeric soluble form of the p75 TNF-α receptor and fractions as a TNF-α antagonist. Its anti-inflammatory effect include several impacts on various cell types, inflammatory pathways, gene activation, nuclear factor kappa B expression, and apoptosis [33].
Non-optical SCM was presently used for monitoring psoriatic plaques in a rapid and non-invasive way. A previous study on untreated psoriasis plaques has suggested some heterogeneity in the SCM presentation [16]. Three main distinct gross levels of capacitance were revealed, each of them probably corresponding to structural and functional differences corresponding to distinct patho biological stages in the evolution of psoriasis [3,16,17]. A vast part of the whitish areas represented low capacitance structures at the skin surface. They probably corresponded to altered corneocyte renewal in hyperkeratotic areas [34]. Such dryer areas created different SCM patterns. Some other fields showed a medium level capacitance tentatively interpreted as parakeratotic foci admixed with neutrophils [16]. These cells were likely more hydrated than the corneocyte cuffing the hyperkeratotic layer. Psoriatic plaques showed further areas exhibiting a third level of much higher capacitance. These sharply circumscribed foci corresponded to erythematous portions of the lesions. These fields possibly fit with the site of superficial vascular hyperplasia and ectasia, and/or to more severe inflammation [3,16,35]. In any case, high capacitance is probably related to serum leakage from the microvasculature, finally steeping the horny layers [20]. Such feature suggests the presence of active pathogenic mechanisms active in psoriasis. It seems that environmental influences play a minimal role in such events [36].
For years, plaque type psoriasis treatment has come a long way from the trial- and-error approach to emerging biotherapy innovation [37]. New tools for investigators and clinicians helped scrutinizing the biological background supporting the therapeutic efficacy [17]. It has proved especially rewarding in providing a means for developing appropriate drugs following rational designs [38].
The present study suggests that the SCM method is sensitive enough to disclose any decrease in inflammation on early biotherapy in evolving psoriatic plaques. The SCM functional method appears more subtle to disclose discrete serous infiltration in the SC than the morphological approach using CSSS. Indeed, SCM measures hydration of the SC which is distinct from the material disclosed by the periodic acid Schiff (PAS) revealed on CSSS. The capacitance level likely represents the initial inflammatory step of psoriasis progression. SCM could be used to the early identification of responders to biologicals. Indeed, the improvement of psoriasis as revealed by the SCM was disclosed in the present study after a one-month treatment that is much shorter than the 12-week period commonly respected for assessing major changes in the PASI score.
Compared with MTX and ETC monotherapies, the combination of both drugs apparently results in increased efficacy with acceptable tolerability and safety in adults with stable moderate to severe plaque psoriasis who was candidates for systemic therapy [39-42]. Bioinstrumental assessments using the present methods have not been yet used for such evaluations. They could be used to compare the information provided by SCM and PASI 75 value at different times of treatment.
Potent systemic therapies are commonly associated with potential cumulative, dose-related toxicities [43]. However, some combination therapies improve the global effects of the drugs [44,45] which could be assessed using SCM and CSSS.
In sum, psoriasis is an IMID with genetic, physiopathologic and clinical heterogeneity. Treatment response varies widely between patients. It is important to identify biomarkers or early clinical evidence to predict treatment response. This is particularly the case for expensive biological therapies.
The present study suggests that SCM and CSSS represent analytical methods helpful for the noninvasive assessment of the effects of MTX, ETC and probably other biologicals in the treatment of moderate-to-severe plaque type psoriasis.
References
- Piérard GE, Piérard-Franchimont C, Szepetiuk G, Paquet P, Quatresooz P. The therapeutic potential of TNF-alpha antagonists for skin psoriasis comorbidities. Expert Opin Biol Ther. 2010 Aug; 10: 1197–1208.
- Mansouri B, Patel M and Menter A. Biological therapies for psoriasis. Expert Opin Biol Ther. 2013 Dec; 13: 1715–1730.
- Piérard GE, Hermanns-Lê T, Piérard-Franchimont C, Piérard SL. Analytical assessment of TNF-antagonist early effects on psoriasis: in vivo real-time reflectance confocal microscopy and skin capacitance mapping. J Med Diagn Meth. 2015; 4: 1000165.
- D’Souza LS and Payette MJ. Estimated cost efficacy of systemic treatments that are approved by the US Food and Drug Administration for the treatment of moderate to severe psoriasis. J Am Acad Dermatol. 2015; 72: 589–598.
- Feldman SR, Zhao Y, Navaratnam P, Friedman HS, Lu J, Tran MH. Patterns of medication utilization and costs associated with the use of etanercept, adalimumab, and ustekinumab in the management of moderate-to-severe psoriasis [Internet]. J Manag Care Spec Pharm . 2015; 21: 201–209.
- Nestle FO, Kaplan DH and Barker J. Psoriasis. N Engl J Med. 2009 Jul 30; 361: 496–509.
- Perera GK, Di Meglio P and Nestle FO. Psoriasis. Annu Rev Pathol. 2012; 7: 385–422.
- Ryan C, Thrash B, Warren RB, Menter A. The use of ustekinumab in autoimmune disease. Expert Opin Biol Ther. 2010 Apr; 10: 587–604.
- Chiricozzi A and Krueger JG. IL-17 targeted therapies for psoriasis. Expert Opin Investig Drugs. 2013 Aug; 22: 993–1005.
- Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008 May; 58: 826– 850.
- Pathirana D, Ormerod AD, Saiag P, Smith C, Spuls PI, Nast A, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009 Oct; 23 Suppl 2: 1–70.
- Smith CH, Anstey A V, Barker JNWN, Burden AD, Chalmers RJG, Chandler DA, et al. British Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2009. Br J Dermatol. 2009 Nov; 161: 987–1019.
- Schäfer I, Hacker J, Rustenbach SJ, Radtke M, Franzke N, Augustin M. Concordance of the Psoriasis Area and Severity Index (PASI) and patient- reported outcomes in psoriasis treatment. Eur J Dermatol. 2010 Jan; 20: 62–67.
- Mattei PL, Corey KC and Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI). The correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol. 2014 Mar; 28: 333–337.
- Griffiths CEM, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010 Jan 14; 362: 118–128.
- Xhauflaire-Uhoda E, Piérard-Franchimont C and Pierard GE. Skin capacitance mapping of psoriasis. J Eur Acad Dermatol Venereol. 2006; 20: 1261–1265.
- Piérard-Franchimont C and Piérard GE. Ustekinumab biotherapy and real-time psoriasis capacitance mapping: a pilot study. J Biomed Biotechnol .2012 Jan; 2012: 870194.
- Delvenne M, Piérard-Franchimont C, Seidel L, Albert A, Piérard GE. The weather-beaten dorsal hand clinical rating, shadow casting optical profilometry, and skin capacitance mapping. Biomed Res Int. 2013; 2013: 913646.
- Piérard-Franchimont C and Piérard GE. Assessment of aging and actinic damages by cyanoacrylate skin surface strippings [Internet] . Am J Dermatopathol. 1987; 9: 500–509.
- Piérard GE, Piérard-Franchimont C, Paquet P, Hermanns-Lê T, Radermacher J, Delvenne P. Cyanoacrylate skin surface stripping and the 3S-Biokit advent in tropical dermatology: a look from Liege. ScientificWorldJournal. 2014; 2014: 462634.
- iérard GE, Courtois J, Ritacco C, Humbert P, Fanian F, Piérard-Franchimont C. From observational to analytical morphology of the stratum corneum. progress avoiding hazardous animal and human testings. Clin Cosmet Investig Dermatol .2015; 8: 113–125.
- Charles J, Chaperot L, Salameire D, Di Domizio J, Aspord C, Gressin R, et al. Plasmacytoid dendritic cells and dermatological disorders: focus on their role in autoimmunity and cancer. Eur J Dermatol. 2010 Jan; 20: 16–23.
- Nickoloff BJ, Xin H, Nestle FO, Qin J-Z. The cytokine and chemokine network in psoriasis. Clin Dermatol .2007 Jan; 25: 568–73.
- Saporito FC and Menter MA. Methotrexate and psoriasis in the era of new biologic agents. J Am Acad Dermatol . 2004; 50: 301–309.
- Nijsten T and Spuls PI. Adalimumab may be better or no worse than methotrexate in the treatment of psoriasis. Br J Dermatol. 2008 Jul; 159: 257–258.
- Barker J, Hoffmann M, Wozel G, Ortonne J-P, Zheng H, van Hoogstraten H, et al. Efficacy and safety of infliximab vs. methotrexate in patients with moderate- to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1). Br J Dermatol. 2011 Nov; 165: 1109–1117.
- Montaudié H, Sbidian E, Paul C, Maza A, Gallini A, Aractingi S, et al. Methotrexate in psoriasis: a systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicity. J Eur Acad Dermatol Venereol . 2011 May; 25 Suppl 2: 12–18.
- Yelamos O and Puig L. Systemic methotrexate for the treatment of psoriasis. Expert Rev Clin Immunol. 2015; 11: 553–563.
- Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003 Nov 20; 349: 2014–2022.
- Gottlieb AB. Etanercept for the treatment of psoriasis and psoriatic arthritis. Dermatol Ther. 2004 Jan; 17: 401–408.
- Papp KA, Tyring S, Lahfa M, Prinz J, Griffiths CEM, Nakanishi AM, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005 Jun; 152: 1304–1312.
- Van de Kerkhof PCM, Segaert S, Lahfa M, Luger TA, Karolyi Z, Kaszuba A, et al. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. Br J Dermatol. 2008 Nov; 159: 1177–1185.
- Griffiths CE, Sterry W, Brock F, Dilleen M, Stefanidis D, Germain JM, et al. Pattern of response in patients with moderate-to-severe psoriasis treated with etanercept. Br J Dermatol. 2015; 172: 230–238.
- Piérard GE, Goffin V, Hermanns-Lê T, Piérard-Franchimont C. Corneocyte desquamation. Int J Mol Med. 2000; 6: 217–221.
- Uhoda I, Piérard GE, Piérard-Franchimont C, Arrese JE, Goffin V, Nikkels A, et al. Vascularity and fractal dimension of the dermo-epidermal interface in guttate and plaque-type psoriasis. Dermatology 2005; 210: 189–193.
- Devillers C, Piérard GE, Quatresooz P, Piérard S. Environmental dew point and skin and lip weathering. J Eur Acad Dermatol Venereol. 2010; 24: 513–517.
- Blauvelt A. New concepts in the pathogenesis and treatment of psoriasis: key roles for IL-23, IL-17A and TGF-β 1. Expert Rev Dermatol. 2007 Feb 10; 2: 69– 78.
- Clemmensen A, Spon M, Skov L, Zachariae C, Gniadecki R. Responses to ustekinumab in the anti-TNF agent-naïve vs. anti-TNF agent-exposed patients with psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2011 Sep; 25: 1037–1040.
- Zachariae C, Mørk N-J, Reunala T, Lorentzen H, Falk E, Karvonen S-L, et al. The combination of etanercept and methotrexate increases the effectiveness of treatment in active psoriasis despite inadequate effect of methotrexate therapy. Acta Derm Venereol. 2008 Jan; 88: 495–501.
- Driessen RJB, van de Kerkhof PCM and de Jong EMGJ. Etanercept combined with methotrexate for high-need psoriasis. Br J Dermatol. 2008 Aug; 159: 460–463.
- Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008 Aug 2; 372: 375–382.
- Gottlieb AB, Langley RG, Strober BE, Papp KA, Klekotka P, Creamer K, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012 Sep; 167: 649–657.
- Piérard-Franchimont C, Piérard GE and Quatresooz P. Focus on skin cancer association and progression under TNF antagonist therapy. Expert Opin Biol Ther. 2011 Sep; 11: 1215–1222.
- Lebwohl M, Menter A, Koo J, Feldman SR. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004 Mar; 50: 416–430.
- Jensen P, Skov L and Zachariae C. Systemic combination treatment for psoriasis: a review. Acta Derm Venereol. 2010 Jul; 90: 341–349.