
Review Article
Austin J Dermatolog. 2017; 4(3): 1078.
Dermatologic Adverse Effects Secondary to EGFR Inhibitors Use as Oncological Treatment: Pathophysiology, Clinical Manifestations and Treatment
Zárraga CA¹*, Vega Memije ME¹ and Rodríguez Cid JR²
¹Department of Dermatology, General Hospital, Mexico City
²Department of Oncology, National Institute of Respiratory Diseases, Mexico City
*Corresponding author: Castañeda Zárraga A, Department of Dermatology, Hospital General “Dr Manuel Gea González”, Calzada de Tlalpan 4800, Tlalpan, CP 14000, Mexico City
Received: June 15, 2017; Accepted: OOctober 17, 2017; Published: October 30, 2017
Abstract
Through the last few years the use of Epidermal Growth Factor Receptor Inhibitors (EGFR) has increased within the treatment of several oncological diseases. Though these drugs offer advantages regarding their systemic adverse events profile, they relate with the appearing of skin lesions among patients. Most frequent are: xerosis, acneiform dermatitis, pruritus, paronychia, and hair alterations. These adverse effects are due to these drugs mechanism of action, and their incidence is a sign of their efficacy.
Regardless the frequency of dermatologic lesions, and the impact these may have on oncological treatment duration, there is still a lack of an established standard treatment or prophylaxis. So far, the treatment is based on antimicrobial agents such as tetracycline and minocycline, topical steroids, anthistaminics, and skin care general measures. This paper reviews dermatologic adverse events of EGFR inhibitors, more common clinical manifestations and different treatments suggested to date.
Keywords: Epidermal Growth Factor Receptor Inhibitors (EGFR); Dermatologic adverse effects; Chemotherapy toxicity
Introduction
Most human epithelial cancers display a marked increase of growing factors, and a particularly high activity of EGFR receptors. When the phenomenon was understood, EGFR became the most important receptor to investigate for researchers, and main therapeutic target for oncological treatment in patients portraying the aforementioned activation [1].
Currently there are two EGFR inhibitor classes for oncological use: monoclonal antibodies and tyrosine kinase inhibitors (TKIs) [1].
Anti EGFR treatments allow for a larger selectivity with better success rates portrayed as response rates, quality of life, progressionfree survival, and overall survival, and for lesser adverse events related to chemotherapy agents [2].
Most common side effects with EGFR treatment are those related to skin, since EGFR activation plays an important role for skin’s innate immunity, and its inhibition reduces the immunity’s integrity. This has been seen in phase II trials with this kind of drugs [3-6] (Table 2).
Although adverse side effects secondary to EGFR inhibition are seldom severe, they do have a high impact on the patient’s quality of life, including physical, financial, emotional, and social aspects [7]. A decrease in dose, and a lack of continuity in treatment have been reported in 60% and 32%, respectively, secondary to dermatological adverse effects caused by EGFR inhibitors, which have diminished therapeutic benefits [2]. Joshi et al observed that emotional impact and detriment in quality of life is greater in patients younger than 50 years old [8].
Pathophysiology
EGFR, also known as ErbB1 (HER1) is part of the tyrosine kinase family of Erb receptors, is a transmembrane protein consisting in an extracellular ligand, a transmembrane region, and an intracellular region located in epithelial origin cells. Inhibiting the signal mediated by EGFR can be achieved using monoclonal antibodies (cetuximab, panitumumab) that bind extracellular domain and prevent ligand binding, or using low molecular weight agents (erlotinib, gefitinib, afatinib) that block triphosphate adenosine binding to receptor’s intracellular portion [9] (Figure 1, Table 1).
Drug
Approved uses
Erlotinib
Non-small cells lung cancer
Pancreatic carcinoma
Gefitinib
Non-small cells lung cance
Afatinib
Non-small cells lung cancer
Cetuximab
Colon and rectal cancer
Squamous cell carcinoma of the head and neck
Panitumumab
Colon and rectal cancer
Table 1: EGFR inhibitors and their approved use in oncological treatment.
Tyrosine Kinase Inhibitors
Drugs
Adverse Effects
Erlotinib
Acneiform dermatitis
Diarrhea
Asthenia
Cough
Dyspnea
Anorexia
Gefitinib
Non-Specific dermatitis
Transaminase elevation
Proteinuria
Diarrhea
Hepatotoxicity
Interstitial lung disease (ILD)
Afatinib
Diarrhea
Acneiform dermatitis
Stomatitis
Paronychia
Xerosis
Anorexy
Pruritus
Vomit
Dyspnoea
Dysphagia
Hipokalemia
Monoclonal Antibodies
Drugs
Adverse Effects
Cetuximab
Nausea
Anemia
Vomit
Neutropenia
Unspecified dermatitis
Asthenia
Diarrhea
Anorexia
Conjunctivitis
Electrolyte disturbances
(hypomagnesemia, hypokalemia, hypocalcemia)
Panitumumab
Acneiform dermatitis
Pruritus
Xerosis
Paronychia
Erythema
Angioedema
Table 2: EGFR inhibitors approved by FDA (2015) and their most common adverse effects [6].
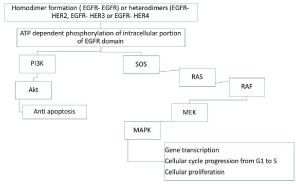
Figure 1: EGFR, HER2, HER3, and/or Her4 receptors are activated,
homodimers or heterodimers are constitute. This starts a phosphorylation
chain ATP dependent of the intracellular domains EGFR specific of tyrosine
kinase. This triggers 2 signaling pathways, the fist (left) activates the anti
apoptotic cascade, and the second (right) activates RAS, RAF, MEK, MAPK
and controls gene transcription from G1 to S and cellular proliferation.
When EGFR is activated, different signaling pathways are produced, of which there are 3 main ones: 1) RAS / RAF /MEK / ERK pathway, with repercussion on cell proliferation, cell cycle progression and migration; 2) Via JAK/ STAT which also promotes cell proliferation, protects against apoptosis and promotes cell motility and 3) PI3K (phosphoinositol 3 kinase) / Akt which acts by inhibiting apoptosis [10].
EGFR is responsible for several skin essential functions throughout development, like epidermal proliferation, differentiation, migration, repairing and carcinogenesis, keeping keratinocytes in an active and proliferative state, in order to promote motility and apoptosis protection [9,11]. EGFR inhibition stimulates pro-inflammatory cytokines CCL2, CCL5 and interleukins. These are important because they are related to dermatitis onset [12].
EGFR participates in the differentiation and development of epidermal keratinocytes, stimulating their growth, inhibiting differentiation, protecting against UV radiation, inhibiting inflammation, and accelerating wounds repairing. It expresses at follicular and epidermal keratinocytes, at the epidermis basal layer, at hair follicle roots, at sebaceous epithelium, at eccrine epithelium, at dendritic cells and other connective tissues [9,12-14].
This process results in a debridement that causes the loss of hydration, and starts xerosis [15]. It is clear that dermatologic damage mechanism started with EGFR inhibition is related with the effects on proliferation and differentiation of keratinocytes promoting secondary inflammation [13].
The mechanism of the effects of EGFR is explained by the inhibition of EGFR that directly affect keratinocytes by inhibiting growth, promoting apoptosis, decreasing cell migration and stimulating inflammation [16]. Acneiform dermatitis is explained by follicular occlusion that occurs as a consequence of the lack of cellular differentiation of the epithelium and release of cytosines leading to inflammation [17], xerosis by the role that EGFR plays in maintaining the integrity of the epidermal barrier; skin fragility and thinning are related to the action of EGFR in the proliferation, differentiation and survival of keratinocytes [18]. Epidermal changes reflect the altered balance between proliferation and differentiation, which may be responsible for xerosis and desquamation observed in some patients treated with Gefitinib [19].
Cell cultures have shown that inhibiting EGFR increases apoptosis levels up to five times between days 4 and 12, which correlates with the clinical appearing of papulopustular skin lesions among patients treated with these drugs [14].
Clinical Manifestations
Different dermatologic adverse effects manifestations have been reported, such as acneiform dermatitis, xerosis, pruritus, hyperpigmentation, eczema, hair damage, paronychia, hand-foot reactions, and even vitiligo [20].
Acneiform dermatitis
The most common dermatologic adverse effect in patients treated with EGFR inhibition, occurring in up to 85% of patients, with a 10%- 20% of them showing severe forms. It has been reported that treatment with monoclonal antibodies and tyrosine kinases inhibitors causes these effects, being most prominent in the first group, particularly with the use of Panitumumab [15,21]. Dermatologic side effects promote treatment cessation in about a 32% of patients, and dose changing in up to 76% of patients [15].
Acneiform dermatitis is characterized by papulopustular lesions, generally accompanied by honey colored scabs and not visible black heads [22]. The most common location is on the face, scalp and upper chest (Figure 2). It has a face flow dissemination, and generally appears during the first month of treatment, and most commonly during first seven to ten days of treatment, though they may appear between days 7 and 42. It generally improves with time, but in only few cases disappears completely while EGFR inhibitors are used [2,23,24]. The lack of open black points distinguishes it from acne; secondary lesions to EGFR therapy are monomorphic, indicating they appeared in a common date [2].
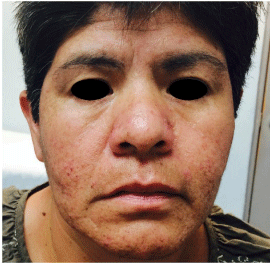
Figure 2: Patient with lung adenocarcinoma treated with afatinib. It can be
seen localized dermatosis on the face, predominantly on the cheeks, chin and
nose with multiple pustules and papules.
Several risk factors have been identified for development of lesions: no smokers, white race, and male sex. Severe forms are more frequent as a consequence of using monoclonal antibodies, and they appear in 10%-17%, when comparing with tyrosine kinases inhibitors with only 5%-9% [15] (Table 3).
Overall dermatologic adverse effects Incidence:
a) Monoclonal antibodies
1) Cetuximab
2) Panitumumab
a) 80% - 100%
1) 88 - 90%
2) 100%
b) Tyrosine kinase inhibitors
b) 43%-54%
Severe dermatologic adverse effects (Grades 3 & 4)
a) Monoclonal antibodies: 10%-17%
10%-17%
b) Tyrosine kinase inhibitors
5%-9%
Table 3: Dermatologic adverse effects and adverse severe effects by drug group incidence.
It has not been seen a relationship between severity and a seborrhea, rosacea or acne background, or dermatologic adverse effects; and when used with RT, the effects are dose dependent [23, 25-28].
Xerosis, pruritus and digital fissures
Xerosis is often observed since the first two months of treatment. At third month, up to a 50% of patients portray it, and at 6 months up to 100% of patients have some degree of xerosis. Skin dryness is usually accompanied by pruritus, which is generalized and present day and night, and it is exacerbated by heat exposition [21]. Elder patients that have received previous therapy with cytotoxic agents and have or have had atopy, usually portray more xerosis [13].
Digital fissures are usually painful, and may impair handy skills [21]. Incidence rate is about 18% in general, and severe forms are about a 3%. Usually they affect finger tips, but they may be seen between fingers, and are related to xerosis [24].
Paronychia
Its characteristic features are painful, edematous and frequently purulent inflammation at the cuticle that can be seen in hands and feet nails, being more common at first fingers. Traumatism is considered an exacerbating factor [15]. It is present in a 12%-58% of cases [24].
It usually appears after six months. It may affect several nails at once, and can be accompanied by onycholysis and lingual pustules (Figure 3). Pain and discomfort frequently require a dose adjustment of oncological treatment [2,21, 23].
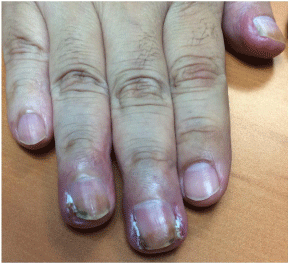
Figure 3: Patient with lung adenocarcinoma treated with afatinib. Paronychia
at third and fourth singers, as well as nails hyperpigmentation.
Hair alterations
Hair manifestations are observed in a 4%-23% of patients, usually appearing after 2-3 months after beginning treatment with EGFR inhibitors. Changes include trichomegaly, facial hair growth, and dry, brittle and fragile hair, as well as no scarring alopecia [21].
Other
Telangiectasia has been observed, usually on the face, behind pinnae, at thorax and extremities, as well as post-inflammatory hyperpigmentation at the areas where acneiform dermatitis and eczema developed, which gets worse with UV radiation [9,29]. Palmoplantar erythrodysesthaesia has been reported as specially related to tyrosine kinase inhibition [30].
Histopathology
At initial stages, inflammatory infiltrate was observed with CD68+ macrophages, CD1+ intraepidermal Lagerhans cells recruitment, and T CD8+ lymphocytes, which usually are not seen in healthy skin. Augmented CD4+ and CD8+ lymphocytes, neutrophil elastase + and mast cells [31].
Similar changes were found at Busam et al trial, where patients were treated with cetuximab therapy, and serial biopsies taken. They began with normal skin and, in subsequent biopsies the first changes seen were T lymphocytes infiltrates, surrounding follicular infundibulum; after a week of treatment, surface perifollicular hyperkeratosis, suppurative folliculitis and focal intraepidermal acantholysis with neutrophils infiltrates involving the terminal portion of the sudoriparous duct. At immunohistochemistry, it was observed that after 8 days of treatment there was 3 to 4 times increment of p27 Kip1 at dermal keratinocytes [26].
Treatment
Side effects importance for treatment resides in the impact of lesions on the patients, since they are cosmetically unpleasant and painful, which jeopardizes both dosing changes and possible treatment continuation. That is the reason for orienting patients about possible adverse effects before the beginning of treatment and starting prophylactic measures to avoid them. Treatment is based in the type of lesions, as well as their severity and extension [13]. Fortunately, dermatologic lesions treatment does not influence patient’s survival [21].
General measures
Treatment begins with skin care general measures and an adequate solar protection. Patients must be oriented to avoid cleaning products that may promote skin dryness. Emollients use may be used in order to avoid xerosis and fissures, but emollients must not be used on the face and trunk, since these can worsen acneiform dermatitis [32].
Acneiform dermatitis
Treatment is based on oral antimicrobials. Low potency steroids (1% hydrocortisone. Level of evidence 2A) may help diminishing pruritus, although potent steroids may aggravate the situation [21]. Topical retinoids and isotretinoin are commonly used, whereas their efficacy has not been proved, as it happens with other topic drugs (metronidazole, clindamycin, benzoyl peroxide, salicylic acid) [13,21].
Segaert et al, proposed as the basis of treatment drugs as metronidazole topical treatment that can be used at 2% cream, applied twice a day. Oral drugs are reserved (Table 4) for clinical cases with a poor response to topical treatment or according to the extent of injuries [32].
Drugs
Dose
Minocycline
100mg/day
Doxycycline
100mg/day
Lymecycline
300mg/day
Table 4: Oral medications recommended for dermatitis acneiform.
Based on frequency, prophylactic management is recommended unless it is contraindicated. Recommended treatment is topical hydrocortisone at 1%, emollients, sunscreen, and doxycycline 100 mg / 12 hours for 6 weeks. Usually doxycycline is preferred over minocycline because of its greater safety, especially in patients with renal failure. Minocycline has shown same efficacy and it is preferred in patients with greater exposition to UV radiation [15].
Xerosis, pruritus and dental fissures
It has been used calamine lotion or menthol 1% for the management of pruritus but this can aggravate xerosis. Other systemic agents such as antihistamines or ?-Aminobutyric Acid (GABA) agonists, gabapentin, pregabalin and doxipentinmay help to control itching [21].
Digital fissures treatment may be carried on with urea emollients at 20%. The risk of developing fissures at feet fingers and heel can be reduced by using socks [21]. Low and medium potency steroids can be used with or without salicylic acid at 10%, as well as propylene glycol at 50% concentration in occluding aqueous solutions (30 minutes daily), cyanoacrylate or hydrocolloids [32].
Paronychia
Prophylactic measures include daily application of topical corticosteroids of medium power in nail folds and baths with antiseptics or diluted chlorine [21]. Paronychia caused by EGFR inhibitors is not infectious, but is very susceptible to become infectious, so it is recommended using at a daily basis creams or soaps with povidone-iodine. Pressure must be diminished by using loose shoes and sox [32].
For established paronychia treatment, it is recommended the use of high potency topical steroids. Systemic antimicrobial must be kept for positive cultures [21].
Hair alterations
Treatment has not been shown to prevent alopecia; recommended actions are support. For non-scarring alopecia, topical minoxidil can be used; for scarring alopecia, there is not any treatment. According to expert opinion topical hydrocortisone can be used at 2% as preventive management [15].
Other manifestations
For the treatment of palmar-plantar erythrodysesthesia the use of emollients containing agents, such as urea at 10%, salicylic acid at 3% or 12% lactic acid is recommended; patient must be informed about the importance of avoiding trauma or pressure by using gloves, special shoes and socks, and not wearing tight jewelry. Steroids such as clobetasol at 0.05% or betamethasone every 12 hours for erythema areas [30].
Conclusions
Adverse effects secondary to EGFR inhibition appear more often on the skin, and affect most of the patients treated with these drugs. This is very important considering patient’s quality of life, as well as the risk that these effects might have on oncological treatment continuation. Pathophysiological mechanisms have not explained totally this phenomenon, and it does not matter if there are trials showing a relationship between their appearance and antitumor activity, results are still little conclusive. To date there are not well established clinical guidelines, and that is the reason for requiring -in a near future- the development of controlled trials in order to know therapeutic utility of side effects.
References
- Ciadiello F, Tortora G. EGFR Antagonist in Cancer Treatment. N Eng J Med. 2008; 358: 1160-1174.
- Lacouture ME, Maitland ML, Segaert S, Setser A, Baran RM, Fox LP. A proposed EGFR inhibitor dermatologic adverse event-specific grading scale from the MASCC skin toxicity study group. Support Care Cancer. 2010; 18: 509-522.
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell-lung cancer. J Clin Oncol. 2003; 21: 2237-2246.
- Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that express the epidermal growth factor receptor. J Clin Oncol. 2004; 22: 1201-1208.
- Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004; 22: 77-85.
- https://www.fda.gov/
- Li Y, Sun H, Xue D. A severe dermatologic adverse effect related with gefitinib: Case report and review of the literature. J Can Res Ther. 2013; 9: 110-113.
- Joshi SS, Ortiz S, Witherspoon JN, Rademaker A, West DP, Anderson R, et al. Effects of Epidermal Growth Factor Receptor Inhibitor-Induced Dermatologic Toxicities on Quality of Life. Cancer. 2010; 116: 1316-1323.
- Mitchel EP, Pérez-Soler R, Van Cutsem E, Lacouture M. Clinical Presentation and Pathophysiology of EGFRI Dermatologic Toxicities. Oncology. 2007; 29: 4-9.
- Pastore S, Lulli D, Girolomoni G. Epidermal growth factor receptor signaling in keratinocyte biology: implications for skin toxicity of tyrosine kinase inhibitors. Arch Toxicol. 2014; 88: 1189-1203.
- Mascia F, Mariani V, Girolomoni G, Pastore S. Blockade of the EGF Receptor Induces a Deranged Chemokine Expression in Keratinocytes Leading to Enhanced skin Inflammation. Am J Pathol. 2013; 163: 303-312.
- Paul T, Schumann C, Rüdiger S, Boeck S, Heinemann V, Kächelle V, et al. Cytokine regulation by epidermal growth factor receptor inhibitor and epidermal growth factor receptor inhibitor associates skin toxicity in cancer patients. Eur J Cancer. 2014; 50: 1855-1863.
- Segaert S, Van Custen E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005; 16: 1425-1433.
- Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Cancer. 2006; 6: 803-812.
- Lacouture ME, Anadkat MJ, Besandoun RJ, Bryce J, Chan A, Epstein JB, et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associates dermatologic toxicities. Support Care Cancer. 2011; 19: 1079-1095.
- Lu PH, Kuo TC, Chang KC, Chang CH, Chu CY. Gefitinib-induced epidermal growth factor receptor-independent keratinocyte apoptosis is mediates by the JNK activation pathway. Br J Dermatol. 2011; 164: 38-46.
- Pérez-Soler R, Delord JP, Halpern A, Kelly K, Krueger J, Sureda BM, et al. HER1/EGFR Inhibitor-Associated Rash: Future Directions for Management and Investigation Outcomes from the HER1/EGFR Inhibitor Rash Management Forum. Oncologist. 2005; 10: 345-356.
- Mascia F, Lam G, Keith C, Garber C, Steinberg SM, Kohn E, et al. Genetic ablation of epidermal EGFR reveals the dynamic origin of adverse effects of anti-EGFR therapy. Sci Tranl Med. 2013; 5: 1-12.
- Lee M, Seo C, Kim S, Yang H, Lee H, Choi J, et al. Cutaneous Side Effects in Non-Small Cell Lung Cancer Patients Treated with Iressa (ZD1839), an Inhibitor of Epidermal Growth Factor. Acta Derm Venerol. 2004; 84: 23-26.
- Nakano K, Komatsu K, Kubo T, Natsui S, Nakui A, Korokawa S, et al. Hand- Foot Skin Reaction is Associated with the Clinical Outcome in Patients with Metastatic Cell Carcinoma Treated with Sorafenib. Jpn J Clin Oncol. 2013; 43: 1023-1029.
- Sinclair R. Anticipating and managing the cutaneous side effects of the epidermal growth factor receptor inhibitors. Asia Pac J Clin Oncol. Melbourne, Australia. 2014; 10: 11-17.
- Pérez-Soler R, Delord JP, Halpern A, Kelly K, Krueger J, Sureda BM, et al. HER1/EGFR Inhibitor-Associated Rash: Future Directions for Management and Investigation Outcomes from the HER1/EGFR Inhibitor Rash Management Forum. Oncologist. 2005; 10: 345-356.
- Fox LP. Pathology and Management of Dermatologic Toxicities Associated With Anti-EGFR Therapy. Cancer Network. 2006; 20: 26-24.
- Porrhaff K, Hofheinz R, Hassel JC, Volkenandt M, Lordick F, Hartmann JT, et al. Interdisciplinary management of EGFR-inhibitor-induced skin reactions: a German expert opinion. Ann Oncol. 2001; 22: 524-535.
- Bianchini D, Jayanth A, Chua YJ, Cunningha, D. Epidermal Growth Factor Receptor Inhibitor-Related Skin toxicity: Mechanisms, Treatment, and its Potencial Role as Predictive Marker. Clin Colorectal Can. 2008; 7: 33-43.
- Busam KJ, Capoiieci P, Matzer R, Kiehn T, Phelan D, Chalpern A. Cutaneous side-effects in cancer patients treated with the antiepidermal growth factor receptor antibody C225. Br J Dermatol. 2001; 144: 1169-1176.
- Tejwani A, Wu S, Jia Y, Agulnik M, Millender L, Lacouture ME. Increased Risk of High-grade Dermatologic Toxicities With Radiation Plus Epidermal Growth Factor Receptor Inhibitor Therapy. Cancer. 2009; 115: 1286-1299.
- Kris MG, Natale RB, Herbs RS, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial. JAMA. 2003; 290: 2149-2158.
- Yang TY, Chen KC, Yin MC, Wang RC, Lin YC. Complications of Therapy in Cancer Patients. J Clin Oncol. 2004; 22: 4646-4648.
- Hofheinz RD, Arnold D, Kubicka S, Prasnikar N, Vogel A. Improving Patient Outcomes with Regorafenib for Metastatic Colorectal Cancer-Patient Selection, Dosing, Patient Education, Prophylaxis, and Management of Adverse Events. Oncol Res Treat. 2015; 38: 300-308.
- Lichtenberg BM, Gerber PA, Holcmann M, Buhren BA, Amberg N, Smolle V, et al. Epidermal EGFR controls cutaneous host defense and prevents inflammation. Sci Tranl Med. 2013; 5: 1-14.
- Segaert S, Van Custem E. Clinical Management of EGFRI Dermatologic Toxicities: The European Perspective. Oncology. 2007; 21: 22-26.