
Research Article
Austin J Nutr Metab. 2020; 7(5): 1095.
The Impact of Nutritional Therapy on Resting Energy Expenditure in Malnourished Older Hospitalized Patients
Pourhassan M*, Daubert D and Wirth R
Department of Geriatric Medicine, Marien Hospital Herne, Ruhr-Universität Bochum, Germany
*Corresponding author: Pourhassan M, Department of Geriatric Medicine, Marien Hospital Herne, Ruhr-Universität Bochum, Germany, Hölkeskampring 40, D-44625 Herne, Germany
Received: June 30, 2020; Accepted: July 30, 2020; Published: August 06, 2020
Abstract
Background and Aims: Little is known about the effect of nutritional therapy on Resting Energy Expenditure (REE) in malnourished older hospitalized patients. We sought to evaluate longitudinal changes in REE during nutritional therapy and to examine the different factors associated with changes in REE among these patients.
Methods: Twenty-three malnourished older patients (age range 67-93, 65% women) participated in this prospective longitudinal observational study. Malnutrition was defined as Mini Nutritional Assessment Short Form (MNA-SF) <8. REE was measured by using indirect calorimetry on hospital admission and at discharge. Body composition (i.e. Fat Free Mass (FFM)) was assessed by bioelectrical impedance analysis. The Parker mobility score was performed to evaluate the patient’s mobility. Nutritional support (i.e. high protein and/or high calorie oral nutritional supplements) was provided to all malnourished patients during hospitalization.
Results: All patients were malnourished with a median MNA-SF score of 6. The median time between two REE measurements was 13 days (interquartile range: 11-15). On admission, REE was significantly lower in patients with lower FFM (P=0.043) and decreased along with the degree of malnutrition (P=0.008). REE (+212.6 kcal, P=0.010) and REE/FFM (+5.6 kcal/kg, P=0.021) increased significantly during hospitalization. In a multiple regression analysis, age, gender and BMI followed by MNA-SF score and mobility were the major independent risk factors of changes in REE.
Conclusion: Low REE in malnourished older patients increased to normal after 2 weeks of nutritional treatment.
Keywords: Malnutrition; Resting Energy Expenditure; Fat Free Mass; Nutritional Therapy; Older Persons
Abbreviations
REE: Resting Energy Expenditure; MNA-SF: Mini Nutritional Assessment Short Form; BIA: Bioelectrical Impedance Analysis; FFM: Fat Free Mass; FM: Fat Mass; SMM: Skeletal Muscle Mass; BW: Body Weight; CRP: C-Reactive Protein; TSH: Thyroid-Stimulating Hormone; IQR: Interquartile Range; SE: Standard Error
Introduction
Malnutrition is a frequent finding in older patients and has commonly a multifactorial etiology. Malnutrition is associated with high risk of complications such as infections, falls, low quality of life, prolonged rehabilitation and hospitalization and higher mortality and morbidity [1]. Although, the prevalence of malnutrition is as high as 50% in hospital admitted older persons [2,3], it remains widely unrecognized and the pathophysiological role and relevance of potential causes are still not well understood [1,4].
It is clear that low energy intake and increased energy requirements are the main mechanisms causing malnutrition [5]. However, malnutrition is often reversible and can be treated with an increase in energy and nutrients provision [6]. For determining the exact energy needs and preventing a negative energy balance, it is of great importance to assess Resting Energy Expenditure (REE). REE is responsible for more than two thirds of Total Energy Expenditure (TEE) and represents the energy needs of an individual to maintain vital organs during rest [7]. REE can be measured by Indirect Calorimetry (IC) which is considered as gold standard.
It is well known that REE declines with age mainly due to decrease in Fat Free Mass (FFM). However, previous studies demonstrated that even after adjustment for FFM and Fat Mass (FM), older individuals have significantly lower REE than younger adults [8,9]. REE may be influenced by other factors such as immobility, inflammation, thyroid hormones and malnutrition. Currently, it remains unclear to what extent this lower REE is a consequence of malnutrition.
A decreased REE in malnourished patients may be a consequence of metabolic adaptation to low energy intake. If this is true, measured REE would be decreased due to malnutrition and may not represent patient’s true energy requirements. It is known that energy deficit and weight loss lead to metabolic adaptation which results in reducing energy expenditure and enhancing metabolic efficiency [10-12]. However, such adaptation may be reversed by nutritional therapy which could lead to an increase in REE and promote body weight regain. Findings of a 6-week subsequent overfeeding-caloric restriction-refeeding study among 32 healthy young men indicated that REE significantly decreased during 3-week caloric restriction due to metabolic adaptation and normalized within 2-week of refeeding [10].
Assessing REE is useful for optimizing and managing nutritional support in malnourished hospitalized patients. However, there is a lack of data on energy needs of malnourished older patients and limited data is available with accurate assessment of total and resting energy expenditure. To the best of our knowledge, there is no study in malnourished older hospitalized patients investigating the effect of nutritional therapy on REE. We hypothesize that the low REE in these patients can be normalized by nutritional treatment. Therefore, the aim of the present study was to evaluate longitudinal changes in REE during nutritional therapy and to examine the different factors associated with changes in REE among malnourished older hospitalized patients.
Materials and Methods
This prospective longitudinal observational study was undertaken between September 2019 - January 2020 at a geriatric acute care unit, at the university hospital, Marien Hospital Herne in Germany. The study participants comprise 23 consecutive malnourished older hospitalized patients with mean age 81.8 ± 8.1 years. Malnutrition was defined as Mini Nutritional Assessment Short Form (MNASF) <8 (13) or weight loss >10% of initial body weight in 6 months or shorter. Other eligibility criteria were age > 65 years, a probable hospital stay of at least 14 days, ability to understand and cooperate and written informed consent. Participants with severe dementia, severe depression, dysphagia, edema, artificial nutrition, i.e. tube feeding and significantly reduced cognitive abilities (Montreal Cognitive Assessment (MoCA) <10) were excluded from the study.
Body weight, REE, activity of daily living and patient’s mobility were conducted during the first days of hospital admission (baseline) and at the time of discharge (follow-up). In addition, body composition analysis and geriatric assessment were performed at hospital admission. Serum concentrations of Thyroid-Stimulating Hormone (TSH) and C-Reactive Protein (CRP) were measured on admission according to standard clinical procedures. CRP level ≥ 2 (mg/dl) was defined as moderate inflammation.
The degree of weight loss was obtained by interview or derived from the patients’ medical records. Furthermore, all malnourished patients received individualized nutritional therapy i.e. high protein and/or high calorie oral nutritional Supplements (ONS) during hospitalization, although, the composition was different based On patient’s Nutritional needs and preferences. The study protocol had been approved by the ethical committee of Ruhr-University Bochum (17-6217, approved on 14.11.2017). Written informed consent was obtained from all patients.
Geriatric assessment
Activities of daily living were determined using Barthel-Index [14]. The point’s range of the German version of the BI is 0-100 pts., with 100 pts. Indicating independency in all activities of daily living. FRAIL scale [15], scoring from 0 (no frail) to 5 (frail), was used to identify persons at risk of frailty. The risk of sarcopenia was screened using SARC-F questionnaire [16], which ranges from 0 to 10, with higher scores representing probable sarcopenia. MoCA [17] was used to evaluate cognitive function with a total score of 30 whereas a score of 26 and higher was considered as normal. Depressive symptoms were diagnosed using Depression in Old Age Scale (DIA-S) [18] with scores 0-2 as having no depression, 3 suspected depression and 4-10 probable depression. The Parker mobility score [19] was performed to evaluate the patient’s mobility indoors, outdoors and during shopping with the total score ranges from 0 to 9. The highest overall score of 9 demonstrates the best possible mobility.
REE
REE was measured by using indirect calorimetry (Q-NRG, Cosmed, Rome, Italy). The device was warmed up before each measurement and flowmeter was calibrated using 3L calibration syringe weekly. In addition, the device was calibrated with a gas mixture of 16% O2, 5% CO2 and balance nitrogen monthly. Clear plastic canopy hood was used for collection of continuous gas exchange measurements for a minimum of ten minutes. The first three minutes of every test were discarded. The measurement of REE was carried in the morning after an overnight fast > 8 h. REE was calculated from whole-body O2 consumption and whole-body CO2 production according to Weir equation [20]. Measured REE was also expressed as a function of FFM (kcal/kg of FFM/day) and body weight (kcal/kg of body weight/day).
Body composition
Measurement of FFM, Fat Mass and (FM), Skeletal Muscle Mass (SMM) of the participants was performed using the phase-sensitive, multi-frequency 8 electrode SECA medical Body Composition Analyser 525 device (BIA, seca mBCA 525, Hamburg, Germany). Measurement was taken in the supine position while 4 pairs of surface electrodes attached 2 to each hand and foot, as described by the manufacturer.
Statistical analysis
All statistical analysis was performed using SPSS statistical software (SPSS Statistics for Windows, IBM Corp, Version 26.0, Armonk, NY, USA). Means and Standard Deviations (SDs) were used for continuous data with normal distribution whereas median values are expressed with Interquartile Ranges (IQR) for non-normally distributed data. Categorical variables are reported as absolute numbers and percentages (n, %). Differences between variables and between baseline and follow-up were analyzed by using paired samples t test for normally distributed values.
Differences in variables at baseline between men and women were analyzed by using an unpaired t test in normally distributed variables and the Mann-Whitney U test for continuous variables with non-normal distribution. Categorical variables were compared by the Chi square test. Pearson’s correlation coefficient was calculated for relations between variables. In addition, multiple regression analysis was performed to investigate the independent effects of age, gender, mobility, BMI, changes in body weight during hospitalization, FFM, inflammation (CRP) and total MNA-SF (as independent variables) on changes in REE as dependent variable. A P-value of <0.05 was considered as the limit of significance.
Results
Baseline characteristics of study participants stratified by gender are summarized in Table 1. Of 23 patients, 65% of subjects were women. All patients were malnourished with a median MNA-SF score of 6. The age range was between 67 and 93 years. The majority of the subjects were frail (91%) and demonstrated an impaired cognitive function (94%) and 48% of the participants exhibited severe depressive symptom.
All (n=23)
Males (n=8; 35 %)
Females (n=15; 65 %)
Age (y)
81.8 ± 8.1
80.2 ± 9.8
82.6 ± 7.3
Height (m)
1.63 ± 0.1
1.70 ± 0.1
1.59 ± 0.1**
Actual body weight (kg)
62.4 ± 11.4
64.7 ± 12.0
61.2 ± 11.3
BMI (kg/m2)
23.4 ± 4.0
22.2 ± 3.0
24.0 ± 4.4
Weight loss in 6 months (kg)
9.5 ± 7.4
13.1 ± 6.8
7.5 ± 7.2
Geriatric assessments, Median (IQR)
MNA-SF
6 (5-6)
5 (4-6)
6 (6-6)
Barthel-Index
45 (40-60)
45 (45-60)
45 (40-60)
Parker mobility score
2 (2-4)
2 (1-5)
2 (2-4)*
Frail Simple scale score
5 (4-5)
4 (3-5)
5 (4-5)
SARC‐F scores
8 (6-9)
6 (5-8)
8 (6-9)
Depression score (DIA-S)
3 (2-6)
4 (2-6)
3 (2-5)
Cognitive function (MOCA)
17 (15-21)
19 (17-22)
16 (14-21)
Bioelectrical Impedance Analysis (BIA)
FM (kg)
18.8 ± 9.6
17.4 ± 10.3
19.5 ± 9.6
FFM (kg)
46.1 ± 7.7
51.6 ± 7.8
43.4 ± 6.3*
SMM (kg)
17.9 ± 4.9
21.0 ± 4.0
16.3 ± 4.5*
CRP (mg/dl)
3.2 ± 2.9
4.3 ± 3.0
2.6 ± 2.8
TSH (mU/ml)
2.1 ± 1.9
1.9 ± 1.1
2.3 ± 2.3
MNA-SF: Mini Nutritional Assessment Short Form; FFM; Fat Free Mass; FM: Fat Mass; SMM: Skeletal Muscle Mass; CRP: C-Reactive Protein; TSH: Thyroid-Stimulating Hormone. Values are given as mean ± SD or median (IQR, interquartile range). *P< 0.05, **P< 0.01 Difference between groups.
Table 1: Characteristic of study population at baseline.
All patients (100%) had probable sarcopenia according to SARC-F and all displayed reduced mobility based on the parker mobility score. Moderate inflammation (CRP> 2 mg/dl) was presented in almost half of the population (49.5%, n=11). Nutritional support (i.e. high protein and/or high calorie ONS) was provided to all malnourished patients during hospital stay although two patients (9%) did not take it at all. All patients were free of either edema or fever.
There were no significant differences in all variables between sexes, except for height, FFM and SMM with lower values in females than males. In addition, men indicated better mobility than women according to the parker mobility score (Table 1).
Results of REE measured by indirect calorimetry at baseline and follow-up and the respective changes are given in Table 2. On admission, REE was significantly lower in patients with lower body weight (r=0.408, P=0.050; Figure 1a), lower FFM (r=0.481, P=0.043; Figure 1b) and decreased along with degree of malnutrition (r=0.541, P=0.008; Figure 1c). In addition, expressed as a ratio of FFM, REE values increased with higher score of MNA-SF on admission however, the association did not reach the level of significance (P=0.762). No significant associations between REE and CRP (P=0.138) and TSH (P=0.967) on admission were found.
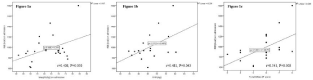
Figure 1: Associations between REE and a) body weight on admission, b) FFM and c) total MNA-SF in total population (n=23). The solid line is the regression line. REE: Resting Energy Expenditure; FFM: Fat Free Mass; MNA-SF: Mini Nutritional Assessment Short Form.
Baseline
Follow-up
Changes
REE (kcal/day)
967.5 ± 260.0
1180.0 ± 397.9
212.6 ± 363.0*
REE (kcal/ kg BW/ day)
15.7 ± 3.8
19.1 ± 6.3
3.4 ± 5.9*
REE (kcal/ kg FFM/ day)
22.0 ± 4.7
27.6 ± 9.9
5.6 ± 9.3*
BW: Body Weight; FFM: Fat Free Mass. *P< 0.01. Difference between baseline and follow-up.
Table 2: Resting Energy Expenditure (REE) measured by indirect calorimetry at baseline and follow-up and the respective changes in total population (n=23).
The median time between baseline and follow-up for REE measurements was 13 days (IQR: 11-15). REE increased significantly during hospitalization (+212.6 kcal, Table 2). In addition, REE/kg BW and REE/kg FFM increased overtime. Moreover, parker mobility score (P <0.001) and Barthel-index (P <0.001) improved during hospital stay. Body weight increased overtime however, the changes did not re ach the level of significance (on admission 62.4 ± 11.4 kg, at discharge 64.1 ± 12.1 kg; P=0.097) mainly due to short duration of the hospitalization. There were no significant differences in total REE values between gender on admission (P=0.722) and at discharge (P=0.551).
In a multiple regression analysis, the effects of age, gender, mobility on admission (parker mobility score), BMI, changes in body weight during hospitalization, FFM, inflammation (CRP) and total MNA-SF score (as independent variables) on changes in REE (as dependent variables) were tested (Table 3). Age, gender female and BMI followed by MNA-SF score and mobility were the major independent risk factors and explained 78.6% of the variance in changes in REE.
Changes in REE
Beta Coefficient
SE
P value
Age
1.23
16.73
0.017
Gender
1.8
46.57
0.032
Parker mobility score on admission
0.8
73.49
0.045
BMI
0.81
29.34
0.045
Changes in body weight*
0.45
18.11
0.126
FFM
1.43
29.3
0.069
Inflammation (CRP)
0.29
60.32
0.354
Total MNA-SF
-2.95
112.1
0.023
REE: Resting Energy Expenditure; FFM: Fat Free Mass; CRP: C-Reactive Protein; MNA-SF: Mini Nutritional Assessment Short Form; SE: Standard Error. *Changes in body weight during hospitalization.
Table 3: Multiple regression analysis of risk factors associated with changes in REE in total population (n=23).
Discussion
Previous cross-sectional and longitudinal studies reported significant decline in FFM with aging [8,21]. Since FFM is the major determinant of REE, explaining 60-90% of its variance [22], REE therefore decreases as a person ages mainly due to the decrease in FFM [8,9]. However, this decrease in REE is prevalent even after adjustment for body composition and must therefore have additional causes.
Findings of the present study indicated that REE is positively associated with FFM and negatively associated with the degree of malnutrition on admission. Indeed, REE was significantly lower in patients with lower FFM and severe malnutrition, suggesting that REE is different even at different stages of malnutrition. In addition, expressed as a ratio of FFM, REE values increased with higher score of MNA-SF however, the association did not reach the level of significance likely due to the small number of subjects. In contrary to our results, in a cross-sectional study of 52 older patients (mean age 79±6 years), Schneider et al. [24] indicated that REE/FFM values increased along with degree of malnutrition (from 32.9 ± 4.1 kcal/kg in patients with BMI 18.5-20 to 44.7 ± 9.5 kcal/kg in those with BMI<16, P=0.001). The discrepant results may be explained by differences in study population and in methods used for assessing nutritional status. In the study by Schneider et al. [24], only malnourished patients with BMI < 20 were included whereas the majority were very lean with BMI between 16-18.5 and < 16 which is very low compared to the mean BMI in our study population (23.4 ± 4.0 kg/m2). Therefore, the very low BMI and relatively low FM in that study may have resulted in a higher mean REE/FFM in severe malnourished patients. In addition, in that study, BMI was used to evaluate the degree of malnutrition however, we assessed nutritional status using MNA-SF, which is considered as a validated tool for screening the nutritional status in older persons across settings and provides true classification of nutritional status compared to the standard BMI.
Energy deficit and weight loss result in metabolic adaptation, which leads to reduced energy expenditure, and may enhance metabolic efficiency [10-12]. Previous studies showed that malnourished patients have a lower REE compared to patients with normal nutritional status [23]. In a cohort study of healthy individuals (age 18-83 years) [24], the mean REE measured by IC in older persons age>70 years was higher compared to the mean measured REE in our malnourished older patients (5 MJ/day=1194 kcal/day vs. 967 kcal/ day, respectively). Accordingly, the low REE in our malnourished patients maybe a consequence of metabolic adaptation to low energy intake. Consequently, REE values may be biased by malnutrition and may not represent patient’s true energy requirements.
The major finding of the present study is that REE, either as absolute values or as a function of FFM, significantly increased after almost 2 weeks nutritional therapy confirming our hypothesis regarding the effect of nutritional treatment on low REE in malnourished persons. It has been previously reported that increased REE or REE/FFM ratio maybe a consequence of hypermetabolic state [25]. It is important to determine that in this study; older patients were free from severe acute stress such as infections. In addition, although, almost half of the population displayed moderate inflammation, no significant associations between REE and CRP on admission were observed and CRP did not explain any variance in changes in REE in regression analysis.
We believe that malnutrition decreases REE as an energy saving component of metabolic adaptation, unless there is a specific cause for an increase in REE such as severe inflammation, whereas nutritional treatment normalizes the low REE in malnourished patients. Indeed, after administration of almost 2 weeks individualized nutritional therapy, mean measured REE by IC increased from 968 to 1180 kcal/ day which was comparable to the mean predicted REE by the Harris- Benedict equation (1190 kcal/day, P=0.901) in healthy persons and to the mean of measured REE by IC reported in previous studies among healthy older individuals [24,26]. In addition, in this study, the parker mobility score and the activities of daily living as measured by Barthel-index improved during hospital stay as the result of routine rehabilitation program in our geriatric acute care unit, i.e. physical and occupational training. Such activities may also increase REE [27], however, the effect appears relatively minor and was not the target of our study.
To the best of our knowledge, this is the first study showing that the low REE in malnourished older hospitalized patients can be reversed by nutritional therapy. Considering these findings, it appears essential to take into account the impact of malnutrition on REE in malnourished older hospitalized patients. In case of doubt, REE estimated by the Harris-Benedict-formula seems to better reflect the true energy needs of these patients than uncorrected data of indirect calorimetry. Furthermore, increasing REE in malnourished older patients seems to be a sign of an effective nutritional therapy.
This study has some limitations. We assessed body composition using BIA, which is highly influenced by hydration status [28]. That is why we excluded subjects with edema. However, BIA can be considered as a validated tool for measuring body composition in hospital settings [29]. We did not measure nutritional intake, which is difficult to perform in a geriatric population. In addition, our study population is relatively small mainly due to difficulty in using indirect calorimetry in some older patients i.e. patients’ fear to use the ventilated canopy hood or patients’ discontent to assess REE two times. However, IC, which is considered as gold standard, was used to determine energy requirements accurately in our patients on admission and at the time of discharge. Further longitudinal research is required to better understand the individual changes of energy needs.
Conclusion
The REE of malnourished older hospitalized patients is lower than expected and predicted by the formula of Harris-Benedict, presumably due to metabolic adaptation. However, low REE increased to normal after 2 weeks of nutritional therapy. The findings of this study indicate that measured REE may not represent the true energy requirements of older malnourished persons.
Author Contributions
The study was designed by MP and RW. MP performed statistical analysis. Data were obtained by DD. MP and RW wrote the manuscript. All authors approved the final manuscript.
Funding
This study was partially funded by the Faculty of Medicine at Ruhr-Universität Bochum through the FoRUM-Program (FoRUM No. F917R-2018).
Bibliography
- Volkert D, Beck AM, Cederholm T, Cereda E, Cruz-Jentoft A, Goisser S, et al. Management of Malnutrition in Older Patients-Current Approaches, Evidence and Open Questions. Journal of clinical medicine. 2019; 8.
- Morley JE. Pathophysiology of anorexia. Clinics in geriatric medicine. 2002; 18: 661-673.
- Pourhassan M, Cuvelier I, Gehrke I, Marburger C, Modreker MK, Volkert D, et al. Risk factors of refeeding syndrome in malnourished older hospitalized patients. Clinical nutrition (Edinburgh, Scotland). 2018; 37: 1354-1359.
- Volkert D, Kiesswetter E, Cederholm T, Donini LM, Eglseer D, Norman K, et al. Development of a Model on Determinants of Malnutrition in Aged Persons: A MaNuEL Project. Gerontology and Geriatric Medicine. 2019; 5: 2333721419858438.
- Volkert D, Kiesswetter E, Cederholm T, Donini LM, Eglseer D, Norman K, et al. Development of a Model on Determinants of Malnutrition in Aged Persons: A MaNuEL Project. Gerontology & geriatric medicine. 2019; 5: 2333721419858438.
- Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet (London, England). 2019; 393: 2312-2321.
- Wickel EE, Eisenmann JC. Within- and between-individual variability in estimated energy expenditure and habitual physical activity among young adults. European Journal of Clinical Nutrition. 2006; 60: 538-544.
- St-Onge MP, Gallagher D. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition (Burbank, Los Angeles County, Calif). 2010; 26: 152-155.
- Frisard MI, Broussard A, Davies SS, Roberts LJ, 2nd, Rood J, de Jonge L, et al. Aging, resting metabolic rate, and oxidative damage: results from the Louisiana Healthy Aging Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2007; 62: 752-759.
- Muller MJ, Enderle J, Pourhassan M, Braun W, Eggeling B, Lagerpusch M, et al. Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota Starvation Experiment revisited. The American journal of clinical nutrition. 2015; 102: 807-819.
- Pourhassan M, Bosy-Westphal A, Schautz B, Braun W, Gluer CC, Muller MJ. Impact of body composition during weight change on resting energy expenditure and homeostasis model assessment index in overweight nonsmoking adults. The American journal of clinical nutrition. 2014; 99: 779-791.
- Trexler ET, Smith-Ryan AE, Norton LE. Metabolic adaptation to weight loss: implications for the athlete. Journal of the International Society of Sports Nutrition. 2014; 11: 7.
- Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Validation of the Mini Nutritional Assessment Short-Form (MNA-SF): a practical tool for identification of nutritional status. The journal of nutrition, health & aging. 2009; 13: 782-788.
- Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. Maryland state medical journal. 1965; 14: 61-65.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. The journal of nutrition, health & aging. 2012; 16: 601-608.
- Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. Journal of cachexia, sarcopenia and muscle. 2016; 7: 28-36.
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005; 53: 695-699.
- Heidenblut S, Zank S. Entwicklung eines neuen Depressionsscreenings für den Einsatz in der Geriatrie. Zeitschrift für Gerontologie und Geriatrie. 2010; 43: 170-176.
- Parker MJ, Palmer CR. A new mobility score for predicting mortality after hip fracture. The Journal of bone and joint surgery British volume. 1993; 75: 797-798.
- Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. The Journal of physiology. 1949; 109: 1-9.
- Fantin F, Di Francesco V, Fontana G, Zivelonghi A, Bissoli L, Zoico E, et al. Longitudinal body composition changes in old men and women: interrelationships with worsening disability. The journals of gerontology Series A, Biological sciences and medical sciences. 2007; 62: 1375-1381.
- Cunningham JJ. Body composition as a determinant of energy expenditure: a synthetic review and a proposed general prediction equation. The American journal of clinical nutrition. 1991; 54: 963-969.
- Schneider SM, Al-Jaouni R, Pivot X, Braulio VB, Rampal P, Hebuterne X. Lack of adaptation to severe malnutrition in elderly patients. Clinical nutrition (Edinburgh, Scotland). 2002; 21: 499-504.
- Geisler C, Braun W, Pourhassan M, Schweitzer L, Gluer CC, Bosy-Westphal A, et al. Age-Dependent Changes in Resting Energy Expenditure (REE): Insights from Detailed Body Composition Analysis in Normal and Overweight Healthy Caucasians. Nutrients. 2016; 8.
- Campillo B, Bories PN, Devanlay M, Pornin B, Le Parco JC, Gaye-Bareyt E, et al. Aging, Energy Expenditure and Nutritional Status: Evidence for Denutrition-Related Hypermetabolism. Annals of Nutrition and Metabolism. 1992; 36: 265-272.
- Bosy-Westphal A, Eichhorn C, Kutzner D, Illner K, Heller M, Muller MJ. The age-related decline in resting energy expenditure in humans is due to the loss of fat-free mass and to alterations in its metabolically active components. The Journal of nutrition. 2003; 133: 2356-2362.
- Weissmann C. The effects of routine intensive care interactions on metabolic rate in ventilated patients1994; Vienna: Springer Vienna. 1984.
- Van Ancum JM, Scheerman K, Pierik VD, Numans ST, Verlaan S, Smeenk HE, et al. Muscle Strength and Muscle Mass in Older Patients during Hospitalization: The EMPOWER Study. Gerontology. 2017; 63: 507-514.
- Cerri AP, Bellelli G, Mazzone A, Pittella F, Landi F, Zambon A, et al. Sarcopenia and malnutrition in acutely ill hospitalized elderly: Prevalence and outcomes. Clinical nutrition (Edinburgh, Scotland). 2015; 34: 745-751.