
Research Article
Austin J Nutr Metab. 2022; 9(2): 1124.
Effects of Standardized Hops (Humulus lupulus L.) Extract on Joint Health: A Randomized, Placebo-Controlled, Double-Blind, Multiple Dose Study
Jäger R1*, Bernier B2, Theodosakis J3, Bonfilio G4, Kerksick CM5 and Purpura M1
1Increnovo LLC, Whitefish Bay, USA
2Ashland LLC, Wilmington, USA
3Theo’s, Inc, Tucson, USA
4Sofar Americas, Inc, Petaluma, USA
5Exercise and Performance Nutrition Laboratory, St. Charles, USA
*Corresponding author: Ralf Jäger, Increnovo LLC, 730 E Carlisle Ave, Whitefish Bay, WI 53217, USA
Received: June 16, 2022; Accepted: July 21, 2022; Published: July 28, 2022
Abstract
Background: This study’s aim was to evaluate the efficacy and safety of 14-days oral supplementation of a standardized hops extract containing 30% alpha acids, Humulus lupulus L. on individuals with osteoarthritis of the knee.
Methods: Thirty-three subjects (26 female, 7 male, 57.0 ± 6.9 years) participated in this randomized, double-blind, multi-dose study. Perceived pain (WOMAC), 20-meter walking performance and clinical safety markers (metabolic panel) was evaluated after 0 and 14 days of standardized hops extract (Perluxan®, 1 g/day [HOPS1G], n = 11 or 2 g/day [HOPS2G], n = 10 or placebo [PLA], n = 12). Changes in WOMAC perceived pain scores from baseline were calculated for all groups and compared against changes observed in PLA. Oneway ANOVA were used to evaluate group differences at each measurement time point. Data in presented as means ± SD. A p-value of 0.05 was used to assess statistical significance.
Results: Pain relief while walking on a flat surface showed significant improvement with HOPS2G two hours after dosing. Additionally, pain was reduced to a greater magnitude in HOPS1G and HOPS2G two and four days after supplementation while changes in HOPS1G after six days were also significantly different than PLA changes. Reductions in pain while lying in bed were significantly greater in HOPS2G three days after supplementation while HOPS1G exhibited greater reductions 12 days after supplementation. Selfreported pain scores while sitting or lying in bed were reduced to a greater magnitude in HOPS1G in comparison to HOPS2G after 6, 7, 8, 10, and 13 days of supplementation.
Conclusion: Supplementation with two different doses of supplementation yielded greater improvements in pain reduction while walking and also demonstrated improvements in the amount that sleep was disrupted due to pain. Self-reported pain levels while sitting or lying in bed exhibited a dosedependent pattern. No clinically meaningful changes in blood or urine markers were noted as a result of supplementation between groups. Supplementation did not appear to impact 20-meter walking performance.
Keywords: Joint health; Hops; WOMAC; Nutritional plant ingredient
Abbreviations
OA: Osteoarthritis; NSAID: Nonsteroidal Anti-Inflammatory Drugs; COX: Cyclooxygenase; VAS: Visual Analog Scale; WOMAC: Western Ontario and McMaster Universities Arthritis Index; PGE2: Prostaglandin E2; CONSORT: Consolidated Standards of Reporting Trial; MSM: Methylsulfonylmethane; SAMe: S-Adenosyl-LMethionine; BMI: Body-Mass-Index; BUN: Blood Urea Nitrogen; ALT: Alanine Amino Transferase; AST: Aspartate Transaminase; ALP: Alkaline Phosphatase
Introduction
Osteoarthritis (OA) is a multi-factorial disease of the joints that requires a variety of pharmacologic and non-pharmacologic interventions to address the highly variable treatment responses among individuals [1]. While many of the world’s rheumatology guidelines for osteoarthritis treatment previously suggested the use of acetaminophen (paracetamol) as a first-line pharmacologic agent, Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) are now considered to be more effective for improving pain and function. NSAIDs function as anti-inflammatory, analgesic, and antipyretic agents. The most commonly used NSAIDs are diclofenac, ibuprofen or salicylates, and are typically used on a regular basis to reduce pain, increase mobility, and improve physical function in arthritis patients [2]. Collectively, NSAIDs inhibit Cyclooxygenase (COX), a key enzyme involved in inflammation, to alleviate pain and suppress inflammation in many forms of arthritis [3]. Prostaglandin E2 (PGE2) is known to be a critical modulator of inflammation and regulation of PGE2 production is dependent upon on COX enzymes [4]. Moreover, arachidonic acid is favorably induced by COX-2 to PGE2 which contributes to inflammation and pain [5]. Therefore, a good NSAID alternative in the form of dietary supplements would comprise bioactive ingredients with high selectivity to inhibit COX-2, which also potently inhibits PGE2 and the production of corresponding human inflammatory cells for the application in osteoarthritis or arthritis.
The cones of the female hops plant (Humulus lupulus L.) have been used as a medicinal plant since Roman times. The whole hop cones as well as hops extracts are used in the brewing industry while the lupulin glands of the hop cones are rich in secondary metabolites including hop bitter acids, volatile oil, and polyphenols [6]. Hop bitter acids, which represent up to 30% of the total lupulin content of hops have been linked to various outcomes related to human health [7,8]. Hop bitter acids consist of alpha-acids or humulones and beta-acids or lupulones which are both characterized as prenylated phloroglucinol derivatives [9].
The antioxidant activity of humulones and lupulones has been demonstrated by efficient radical scavenging activity as well as potent inhibition of lipid peroxidation [10]. Hops extract has also shown to possess inhibitory effects on nitric oxide induced by a combination of lipopolysaccharide and IFN-γ (Interferon gamma) which plays an important role in many inflammatory responses [11]. Humulone isolated from hops extract has previously been shown to suppress TNF-a and COX-2 gene transcription [12]. In addition, Lemay et al. [13] showed that hops extract exhibited COX-2 inhibition over ninehour periods in a manner that was similar to ibuprofen in addition to significant sparing activity of COX-1. Similar results were obtained by Hougee et al. [14] by using a standardized carbon dioxide hops extract in an in vitro model. Interestingly, the extract from Humulus lupulus L. potently inhibited PGE2 production of human inflammatory cells through COX-2 inhibition. In consideration of these findings, hops extract may represent a nutritional alternative to improve joint health and mobility in healthy subjects with osteoarthritis. Despite the previous use of hops in this manner, controlled clinical studies on the efficacy of hops on joint health in osteoarthritis populations are currently lacking. Beyond additional research, the need to understand what dose (if any) offers the greatest potential to mitigate pain while minimizing adverse events or other safety concerns is an important area of future research. In addition, the current lack of research also creates the need for well-controlled investigations that have evaluated the safety potential which can be expected from consuming a hops extract. For these reasons, the purpose of this study was to examine the impact of 14 days of oral supplementation of two doses of a standardized hops extract on joint pain, physical function, and clinical safety biomarkers in individuals with osteoarthritis of the knee.
Materials and Methods
Experimental Design
A randomized, double-blind, placebo-controlled, multiple dose study with a parallel design was performed. The study was retrospectively registered with the ISRCTN registry as ISRCTN10531169 and a Consolidated Standards of Reporting Trial (CONSORT) diagram for all study recruitment randomization, and project completion is provided as (Figure 1).
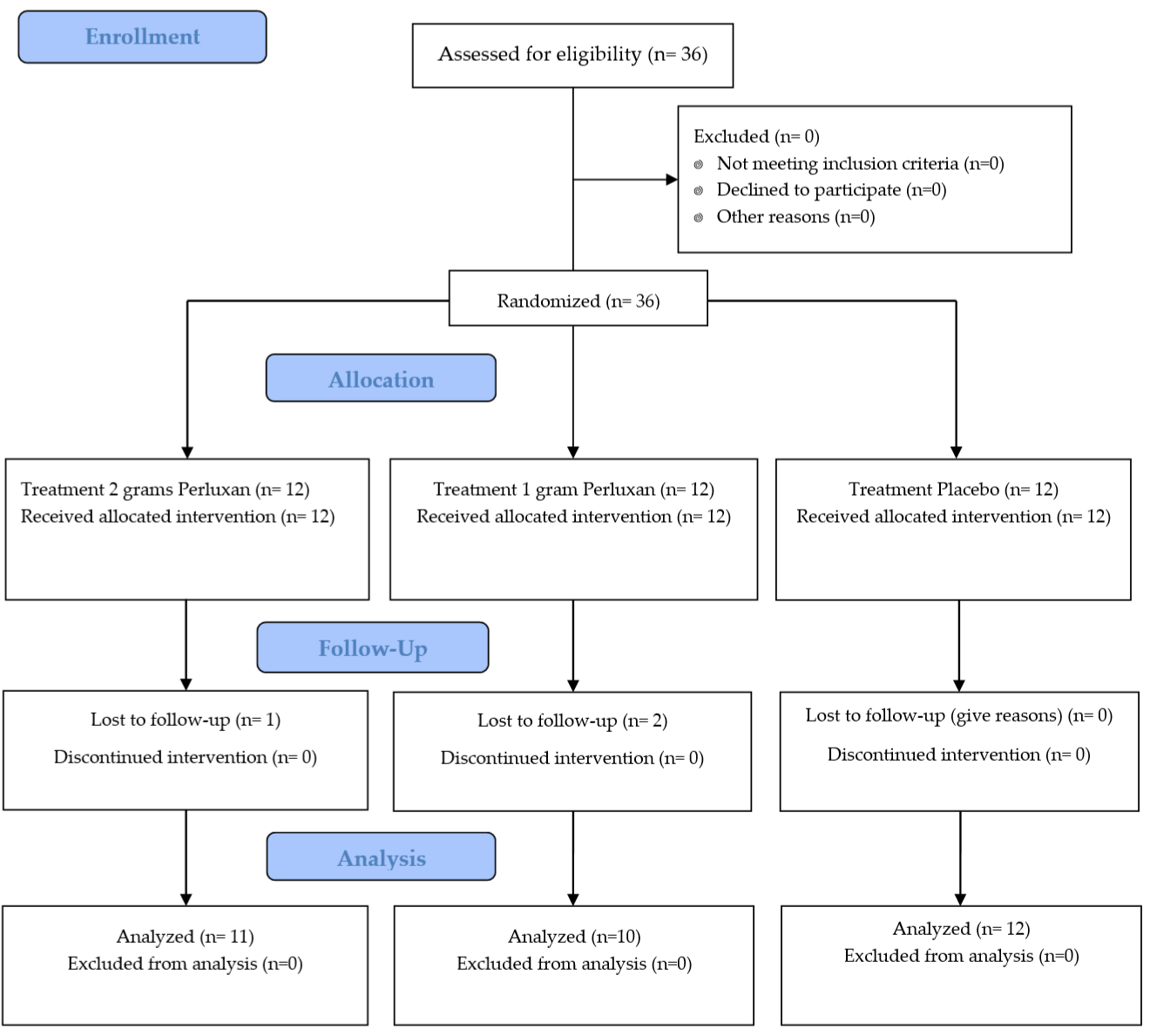
Figure 1: Consolidated Standards of Reporting Trial (CONSORT) outlining recruitment, screening, randomization, and project completion.
Subjects were required to report twice to the study site (Day 1 and Day 15) and prior to both visits were instructed to fast overnight (10 to 12 hours) while maintaining adequate hydration as indicated in (Figure 2).
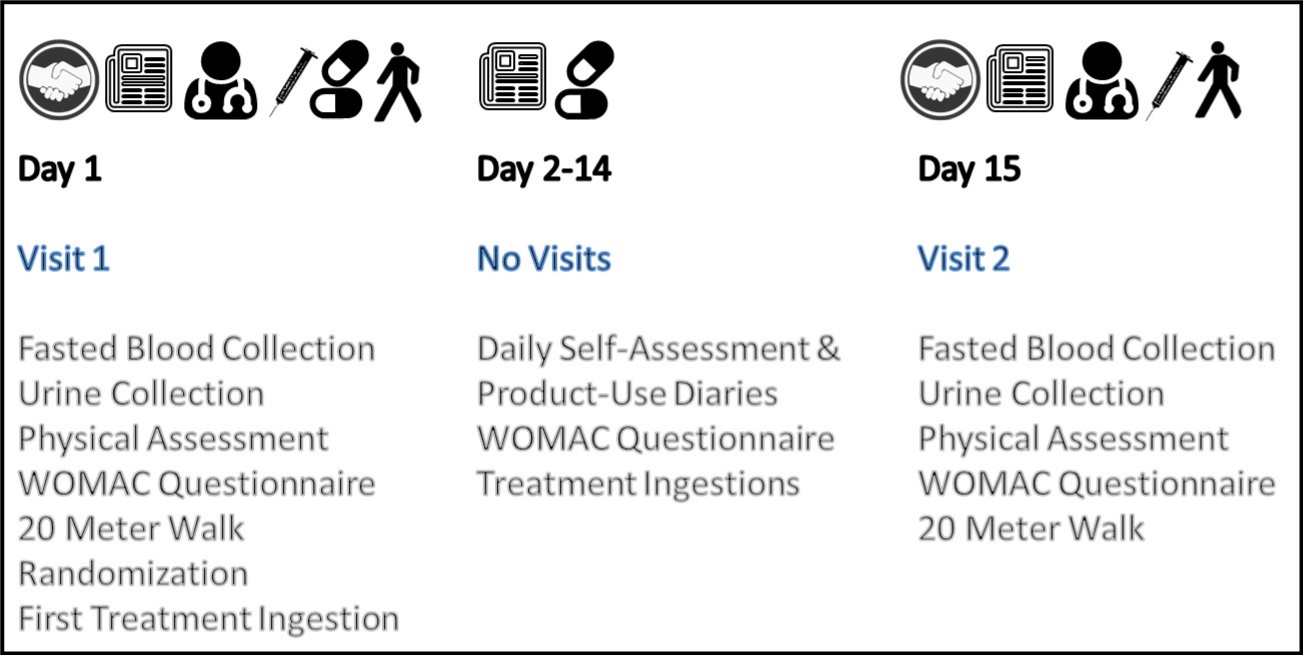
Figure 2: General outline of study activities for the entire study protocol.
On Day 1 (pre-test) subjects read and signed informed consent documents, completed medical histories, and provided information pertaining to concomitant medications. Height, weight, body mass index, and vital signs (blood pressure, heart rate, and body temperature) were measured. A physical assessment of the knees was conducted, at which the investigator confirmed the target knee selected. Perceived pain of the target knee was assessed using a visual analog scale (VAS, 100mm scale with ratings between 0 (no pain) and 100 (very severe pain)) following a timed walk on a flat surface for 20 meters. A fasting blood sample was collected to analyze levels of clinical safety parameters and a urine sample was collected to assess creatinine and oxidative stress. Once deemed eligible, participants were randomized into one of three different treatment groups. A baseline Western Ontario and McMaster Universities Arthritis Index (WOMAC) 5-item Symptom Assessment Questionnaire was completed and the assigned study product, sufficient to last until the next visit, was dispensed, along with written instructions for product use. The first dose was administered at the study site. Question 1 of the WOMAC 5-item Symptom Assessment Questionnaire, perceived pain walking on a flat surface was recorded 1, 2 and 4 hours after ingestion of the first dose of supplementation. Subjects, who currently used NSAIDs or any other anti-inflammatory or pain medication, were instructed to discontinue taking any such products during the remainder of the study. Subjects were instructed that, if necessary, up to 2,000 mg of acetaminophen, was allowed to be taken for arthritis and other pain, but for no more than two days per week (rescue medication). The use of acetaminophen had to be discontinued within two hours after intake of the study product and within 48 hours prior to visit 2. Subjects were instructed to record any use of acetaminophen, the amount taken, and the reason it was taken in a self-assessment and product use diary. To aid in compliance and optimize the ecological validity of the study investigation, study participants were asked to complete the WOMAC 5-item Symptom Assessment Questionnaire at home on a daily basis throughout the entire 14-day supplementation protocol. Subjects were instructed to bring all unused study product, the study product containers, and the diaries with them to the post-test and were instructed to fast at least 10 hours before the post-test and not to take any study product on that day.
The subjects reported back to the study site on day 15 (posttest). Study product was collected and compliance with dosing regimen was determined by capsule count and dosing diary review. Information pertaining to potential adverse events, concomitant illnesses (including the worsening of any intercurrent illnesses), and changes in or additions to concomitant medications was reviewed and recorded. The use of rescue medication was recorded by diary review. Vital signs were measured, and fasting blood and urine samples were collected. Perceived pain of the target knee was assessed using a VAS scale following a walk on a flat surface for 20 meters, and the time to perform a 20-meter walk on a flat surface was recorded.
Study Participants
Thirty-six subjects meeting the criteria of osteoarthritis of the knee according to the American College of Rheumatology (ACR), Class I, II or III, without overt clinical signs of inflammation (i.e. joint swelling, effusion, erythema, palpable increased warmth), were enrolled in the study. Due to a lack of previous research that has examined the impact of hops supplementation on pain outcomes, a moderate effect size of 0.25 was used to determine sample size. Consequently, it was determined that at an alpha level of 0.05, power of 0.70 and an effect size of 0.25, that a total sample size of 33 was required. An additional three participants were subsequently recruited to account for attrition. Inclusion criteria included male or females who were 40 to 75 years of age; VAS pain score of ≥ 30 to ≤ 80 on question 1 (pain walking on a flat surface) of the 5-item Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) assessment questionnaire; a Body-Mass-Index (BMI) of < 39.9 kg/m2; pain in the target knee for at least six months prior to the study start and must be able to walk unassisted (the use of a walking stick, crutch, and/or knee brace is permitted, at the discretion of the investigator). Exclusion criteria included any inflammatory arthritis, or any acute joint trauma at the knee with OA; any signs of active target joint inflammation including redness, warmth, and/or a large, bulging effusion of the target knee joint with the loss of normal contour of the joint at the baseline examination; secondary OA of the study target joint and/or musculoskeletal disease; morning stiffness of greater than 30 minutes duration; knee replacement surgery of the target joint; any active malignancy of any type or history of malignancy; any clinically significant abnormal screening laboratory test values, at the discretion of the principal investigator; history of cardiac event; coronary artery disease or suffers other clinical manifestations of atherosclerotic disease; history of coronary artery bypass graft surgery; current asthma; allergy to ibuprofen, aspirin, acetaminophen, or any NSAID medications; chronic pain syndrome in the judgment of the principal investigator, and has shown minimal response to any analgesic or anti-inflammatory medication; receiving therapy for chronic pain conditions for indications other than OA; class IV unilateral or bilateral OA of the knee, according to the ACR criteria; taking glucosamine, with or without chondroitin sulfate, Methylsulfonylmethane (MSM), SAMe (S-Adenosyl-L-Methionine) and/or shark cartilage, who has not been stabilized on therapy within the three-months prior to the screening evaluation, or who does not expect to remain on the same or comparable medication and corresponding dose; any analgesic or anti-inflammatory drug(s) within five drug half-lives prior to dispensing study materials; hypolipidemic agents; blood pressure medications called angiotensin converting enzyme inhibitors and/or proton pump inhibitors; any medication which inhibit platelet aggregation and/ or anticoagulant drugs; antidepressants, unless stable three months prior to enrollment; history or the current presence of stomach (peptic) ulcer disease or stomach bleeding; history or the current presence of uncontrolled metabolic disorders, thyroid disease, diabetes mellitus type I or II, renal disease, hepatic disease, and/or untreated hypertension; chronic disease which limit his/her activities; history or the current presence of significant hematological disorders; intra-articular, oral and/or parenteral corticosteroids, within the two months prior to study enrollment or plans to receive such therapies during the study period; hyaluronic acid injections in the target joint, within six months of randomization; body mass index > 39.9 kg/m²; woman who is pregnant; known allergy or sensitivity to any of the test product’s ingredients; history of smoking, alcohol and/or other drug abuse, within the past year; and any findings on physical examination, in results of laboratory analyses and/or any medical conditions or concomitant medications or supplements which, in the opinion of the investigator, may place the individual at risk and/or confound the results of the study.
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)
To assess changes in pain, stiffness, discomfort, and physical function, the WOMAC was administered to all participants throughout the study protocol. The WOMAC is a self-evaluation instrument of hip and knee osteoarthritis that has been widely used in the scientific literature [21,22]. It consists of 24 items that are divided into three subscales representing pain, stiffness, and physical function. Each question is rated on a 5-item scale with potential answers being: None, Mild, Moderate, Severe, and Extreme. For this study, only questions from the pain scale were used. All questions were asked in reference to the amount of pain felt in the hips or knees during the last 48 hours. Question 1 related to pain experience while walking on a flat surface. Question 2 related to pain while going up and down stairs. Question 3 was in reference to how pain disturbs sleep at night while in bed. Question 4 was in reference to pain experienced while sitting or lying. Question 5 was centered on pain while standing upright. Baseline (pre-supplementation) WOMAC values were first assessed and then question 1 of the WOMAC (Perceived pain while walking on a flat surface) was asked again one, two, and four hours after taking the first supplementation dose. From there, participants continued to supplement as indicated and the WOMAC administered each subsequent day of the 14-day supplementation protocol.
20-Meter Walking Performance
The 20-meter walk test was performed before and after the supplementation protocol. Participants completed the 20-meter walk test in a long, unobstructed hallway. All participants wore comfortable, soft-soled shoes and did not use walking aids. Once the participant started walking, the timer was started, and the assessor stopped timing when the participant’s first heel completely crossed the finish line. The reliability of the 20-meter walk test has been previously published [14].
Venous Blood and Urine Collection
Study participants had a venous blood sample collected from a forearm vein after observing an overnight fast at the beginning and end of supplementation. Venous blood was collected into an Ethylenediaminetetraacetic Acid (EDTA) and serum (nontreated) collection tube. All tubes, upon collection, were gently inverted and sat at room temperature until being centrifuged (4000 rpm for 20 minutes at 4oC) and processed into their plasma and serum components, respectively. After centrifugation, samples were shipped to a diagnostic laboratory (Core Laboratories, 10 Nevada Drive, Lake Success, NY 11042, USA) where glucose, Blood Urea Nitrogen (BUN), creatinine, total protein, albumin, total bilirubin, Alanine Amino Transferase (ALT), Aspartate Transaminase (AST) and Alkaline Phosphatase (ALP) were measured. Mid-flow urine samples were collected by all study participants upon reporting to the laboratory. All urine samples were assayed for urinary creatinine and isoprostanes (IPF-2 alpha-III, and IPF-2 alpha VI), markers of lipid peroxidation by a diagnostic clinical laboratory (Core Laboratories, 10 Nevada Drive, Lake Success, NY 11042, USA) [22].
Supplementation Regimen
Qualified subjects were assigned in random order to ingest either 1 g per day (HOPS1G, n=12), or 2 g per day (HOPS2G, n=12) of a standardized supercritical carbon dioxide hops extract containing 30% alpha and iso-alpha acids (humulone, cohumulone) (Perluxan®, Pharmachem Laboratories LLC, Kearny, NJ, USA), or a matching maltodextrin placebo (PLA, n=12). The supplements were administered in gelatin capsules that were of similar shape, size, color, and transparency and were packaged into a blindly labeled containers. The supplements were taken as a split dose with or without food in the morning, and the second dose in the evening at least 10-12 hours after the morning dose. The 14-day supplementation period was started immediately after the pre-test to assess current pain (baseline) and was continued until the day before the post-test.
Statistics
All data are presented as means ± standard deviations. Outcomes were considered statistically significant when the probability of type I error (p) was 0.05 or less. Missing WOMAC values were replaced using an intent-to-treat approach. In this respect, four values were replaced from PLA from three participants, two values from two participants in HOPS1G, and two values from two participants in HOPS2G. All values replaced were for either day 13 or 14 of supplementation as no other data was missing. WOMAC outcomes (absolute scores) were assessed using two approaches. Mixed (3 x 15) factorial ANOVA with repeated measures on time were completed to evaluate main effects for time and group as well as their interaction. In addition, a key consideration for our findings was pain relief direction in comparison to PLA and how many days after supplementation did significant pain relief occur (if at all). As a result, separate one way ANOVA with Tukey post-hocs were assessed for each WOMAC variable to assess pain differences at each day of supplementation. Changes in blood and urine markers of safety were assessed using 3 x 2 (group x time) mixed factorial ANOVA with repeated measures on time were performed to evaluate main effects for group and time and the group x time interaction effect. Within group changes across time were assessed using separate paired samples t-tests. Percentage changes from baseline were computed and are presented along with relevant p-values. Figures were produced using Microsoft Excel (Seattle, WA). A total sample size of 33 participants was determined to be needed at an alpha level of 0.05, power (beta) of 0.70, and an effect size of 0.25.
Results
Participant Demographics
Thirty-three subjects, age 39 to 74 years completed the study. The average age of the subjects was 57.3 ± 7.1 years with 92% of them Caucasian (92%) and 78% female. There were no significant differences (p > 0.05) among groups on these demographics (Table 1).
PLA
HOPS1G
HOPS2G
All Subjects
(n=12)
(n=10)
(n=11)
(n=33)
Age (years)
Mean ± SD
56.7 ± 7.8
55.6 ± 5.6
59.6 ± 7.5
57.3 ± 7.1
Range
39 - 74
46 - 64
48 - 74
39 - 74
Race n (%)
Caucasian
11 (92%)
11 (92%)
11 (92%)
33 (92%)
Black
0 (0%)
1 (8%)
1 (8%)
2 (5%)
Hispanic
1 (8%)
0 (0%)
0 (0%)
1 (3%)
Gender (%)
Female
11 (92%)
10 (83%)
7 (58%)
28 (78%)
Male
1 (8%)
2 (17%)
5 (42%)
8 (22%)
Table 1: Baseline demographics (age, gender, and race).
Safety and Adverse Events
Comparison of vital signs (blood pressure, heart rate, and body temperature), glucose, Blood Urea Nitrogen (BUN), creatinine, total protein, albumin, total bilirubin, Alanine Amino Transferase (ALT), Aspartate Transaminase (AST) and Alkaline Phosphatase (ALP), and urinary levels of creatinine, IPF-2 alpha-III, and IPF-2 alpha VI were made before and after supplementation. With the exception of albumin, no baseline differences (p > 0.05) were identified between any groups for any parameters. Albumin levels in HOPS2G (Table 2) were greater than HOPS1G (p < 0.05), but all reported values remained within clinical norms. Similarly, urinary IPF-2 Alpha VI exhibited a significant group x time interaction with HOPS1G increasing to a greater degree than PLA or HOPS2G from day 1 to day 15. In this instance, the mean values for HOPS1G were lower than PLA and HOPS2G at day 1 and increased to day 15 levels that were similar to PLA and HOPS2G. As a result, these outcomes were deemed to be clinically insignificant. No other significant group x time interaction effects were realized (Table 2). Two subjects in PLA and one subject in HOPS2G experienced moderate adverse events (PLA: right shoulder pain, headache, intermittent lower back pain; HOPS2G: flu symptoms, fever, vomiting, laryngitis) during the study and the events were deemed to have no relationship to the treatment. One adverse event in HOPS2G (mild, intermittent belching) was deemed to have a possible relationship to the treatment. All adverse events were resolved by the end of the study.
Variables
N
Baseline
Post
Effect
p-value
Glucose (mg/dL)
PLA
12
95.7 ± 6.5
94.3 ± 8.6
Group
0.99
HOPS1G
10
96.0 ± 17.5
95.0 ± 17.3
Time
0.92
HOPS2G
11
93.7 ± 10.8
96.5 ± 9.7
G x T
0.41
Blood Urea Nitrogen (mg/dL)
PLA
12
16.50 ± 5.11
16.42 ± 4.10
Group
0.71
HOPS1G
10
18.18 ± 6.43
17.09 ± 5.87
Time
0.85
HOPS2G
11
17.58 ± 4.32
18.42 ± 3.82
G x T
0.42
Creatinine (mg/dL)
PLA
12
0.85 ± 0.12
0.84 ± 0.11
Group
0.07
HOPS1G
10
0.91 ± 0.16
0.89 ± 0.12
Time
0.09
HOPS2G
11
0.99 ± 0.14
0.95 ± 0.13
G x T
0.74
Total Protein (mg/dL)
PLA
12
7.55 ± 0.36
7.25 ± 0.43
Group
0.58
HOPS1G
10
7.62 ± 0.45
7.33 ± 0.59
Time
0.001
HOPS2G
11
7.41 ± 0.42
7.21 ± 0.23
G x T
0.8
Albumin (mg/dL)
PLA
12
4.60 ± 0.17
4.41 ± 0.26
Group
0.08
HOPS1G
10
4.71 ± 0.15
4.50 ± 0.25
Time
<0.001
HOPS2G
11
4.56 ± 0.13
4.33 ± 0.23
G x T
0.91
Bilirubin (mg/dL)
PLA
12
0.59 ± 0.27
0.57 ± 0.34
Group
0.95
HOPS1G
10
0.61 ± 0.29
0.49 ± 0.23
Time
0.006
HOPS2G
11
0.65 ± 0.24
0.51 ± 0.29
G x T
0.3
Aspartate Aminotransferase-AST (U/L)
PLA
12
19.6 ± 3.7
19.3 ± 1.92
Group
0.02
HOPS1G
10
27.8 ± 9.4
28.0 ± 13.2
Time
0.91
HOPS2G
11
24.6 ± 6.2
24.3 ± 5.2
G x T
0.98
Alanine Aminotransferase-ALT (U/L)
PLA
12
20.3 ± 6.8
20.1 ± 5.8
Group
0.07
HOPS1G
10
27.6 ± 11.3
29.7 ± 13.4
Time
0.51
HOPS2G
11
26.5 ± 10.0
26.8 ± 8.3
G x T
0.67
Alkaline Phosphatase (U/L)
PLA
12
78.5 ± 17.1
76.3 ± 16.3
Group
0.12
HOPS1G
10
81.8 ± 12.4
81.1 ± 10.3
Time
0.11
HOPS2G
11
70.5 ± 14.9
68.0 ± 14.4
G x T
0.79
Urine Creatinine (μmol/kg/24 hours)
PLA
12
115.6 ± 48.3
114.1 ± 51.8
Group
0.25
HOPS1G
10
93.7 ± 46.6
109.0 ± 64.0
Time
0.05
HOPS2G
11
147.3 ± 74.6
138.4 ± 82.1
G x T
0.82
Urine IPF2 Alpha III Isoprostane (ng/mg creatinine)
PLA
12
0.25 ± 0.17
0.21 ± 0.16
Group
0.62
HOPS1G
10
0.27 ± 0.30
0.34 ± 0.39
Time
0.21
HOPS2G
11
0.19 ± 0.14
0.40 ± 0.37
G x T
0.26
Urine IPF2 Alpha VI Isoprostane (ng/mg creatinine)
PLA
12
1.74 ± 0.74
1.61 ± 0.80†
Group
0.42
HOPS1G
10
1.24 ± 0.68
1.51 ± 0.55
Time
0.89
HOPS2G
11
1.37 ± 0.84
1.20 ± 0.93†
G x T
0.05
†= Change in group from baseline is different than HOPS1G (p < 0.05).
Table 2: Blood and Urine Clinical Markers of Safety.
Use of Rescue Medication
Four subjects used rescue medication (acetaminophen) a total of five times. Three subjects in PLA used rescue medication once (on days 1 (shoulder pain), 6 (headache), and 14 (back pain), respectively) and one subject in HOPS2G (on days 7 and 11 (shoulder pain) used rescue medication twice.
WOMAC – Pain While Walking on a Flat Surface
Figure 3A illustrates the changes in self-reported pain levels from all study participants one, two, and four hours after completing the 20-meter walking test. One way ANOVA using baseline values indicated no statistically significant difference in baseline values between groups (p = 0.11). Two hours after ingesting their first supplementation dose and when compared to PLA, HOPS2G resulted in statistically significant (p < 0.05) improvements in WOMAC Pain While Walking on a Flat Surface values while changes in HOPS1G tended to (p < 0.1) yield improvement of mean pain relief. When viewed collectively and analyzed using a 3x4 mixed factorial ANOVA, a significant time effect (p < 0.001) was observed while no significant group x time interaction was realized (p = 0.13). (Figure 3B) illustrates the changes in self-reported pain levels from all study participants 2 – 7 days beginning supplementation. In comparison to PLA, self-reported pain levels were improved in HOPS2G two and four days after supplementation with a trend towards improved self-reported pain after three days. In comparison to PLA, HOPS1G reported significant (p < 0.05) improvement in mean pain relief two, four and six days after supplementation. Mixed factorial ANOVA indicated a significant time effect (p < 0.001) while no significant group x time interaction was realized (p = 0.79). Figure 3C illustrates the changes in self-reported pain levels from all study participants 8 – 14 days beginning supplementation. No changes were observed between groups over this time period.
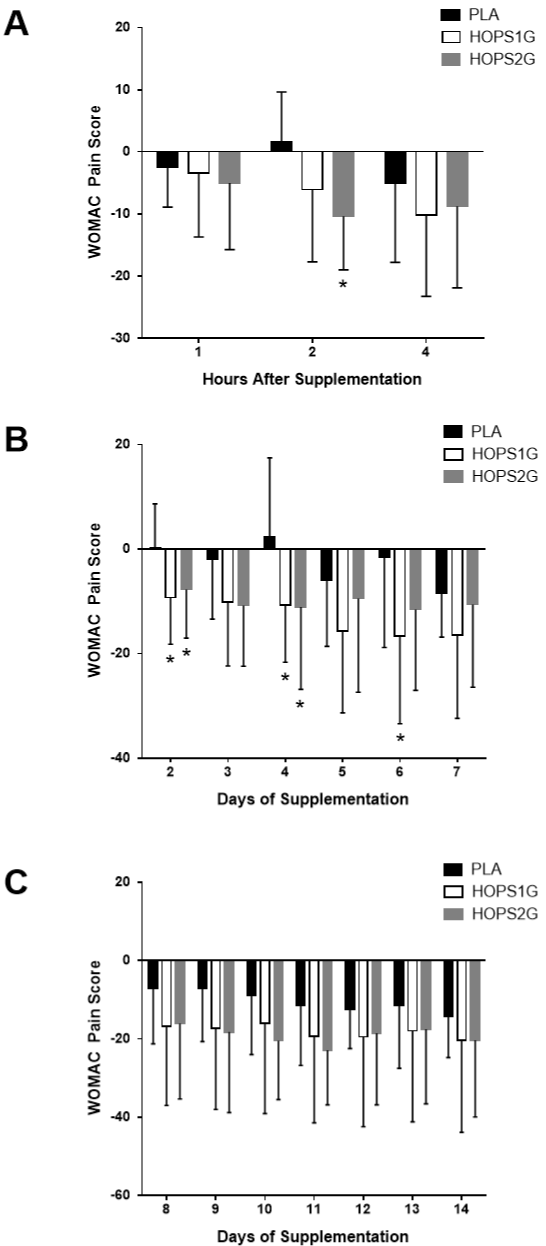
Figure 3: WOMAC Pain While Walking on a Flat Surface. Panel 3A represents
self-reported changes one, two, and four hours after completing the 20-meter
walking test. Panel 3B represents self-reported changes in pain 2 – 7 days
after beginning supplementation. Panel 3C represents self-reported changes
in pain 8 – 14 days after beginning supplementation. Data is presented as
means ± SD. * indicates a difference (p < 0.05) from PLA.
WOMAC – At Night While Lying in Bed, Pain Disturbs Sleep
Figure 4 A illustrates that HOPS2G showed significant (p < 0.05) reductions in reported pain levels, in comparison to PLA, on day 3 while reductions observed on day 2 and 13, respectively tended (p < 0.1) to be different than the changes observed in PLA. Mean scores for HOPS1G were significantly (p < 0.05) reduced, in comparison to PLA, on days 12 and 13 (Figure 4B), while reductions on days 3, 5, 7, 8, and 11 all tended (p < 0.1) to be reduced to a greater than what was observed in PLA. Mixed factorial ANOVA (3x15) with repeated measures for time indicated a significant main effect for time (p < 0.001) and no significant group x time interact (p = 0.22). One way ANOVA indicated no differences (p = 0.14) in baseline values.
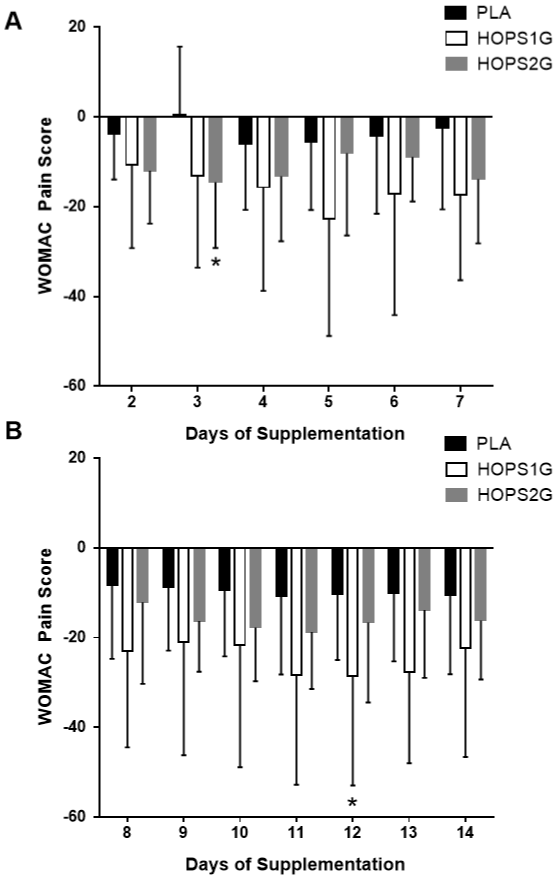
Figure 4: WOMAC – At Night While Lying in Bed, Pain Disturbs Sleep.
Panel 4A represents self-reported changes in pain after 2 – 7 days of
supplementation. Panel 4B represents self-reported changes in pain 8 – 14
days after beginning supplementation. Data is presented as means ± SD. *
indicates a difference (p < 0.05) from PLA.
WOMAC – Pain While Sitting or Lying
HOPS1G exhibited improvements of mean pain relief that were significantly greater than changes observed in HOPS2G on days 6, 7, 8, 10 and 13 while changes observed between HOPS1G and HOPS2G tended (p < 0.1) to be different on days 5, 9, 11, 12 and 14 (Figure 5). No changes in comparison to PLA were observed. Mixed factorial ANOVA (3x15) with repeated measures for time indicated a significant main effect for time (p < 0.001) and no significant group x time interaction (p = 0.19). Oneway ANOVA indicated no differences (p = 0.39) in baseline values.
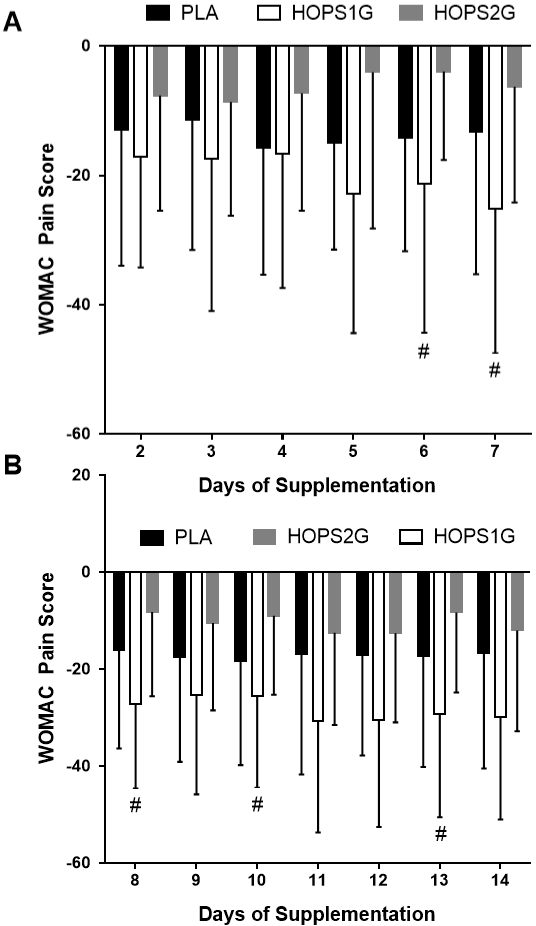
Figure 5: WOMAC – Pain While Sitting or Lying. Panel 3A represents
self-reported changes in pain after 2 – 7 days of supplementation. Panel
3B represents self-reported changes in pain 8 – 14 days after beginning
supplementation. Data is presented as means ± SD. # indicates a difference
(p < 0.05) from HOPS2G.
WOMAC – Pain While Standing Upright
No statistically significant changes in mean pain relief when standing upright were identified. Mean changes for each group do highlight that HOPS1G showed the greatest improvements when compared to HOPS2G and PLA during all test days. No changes in comparison to PLA were observed. Mixed factorial ANOVA (3x15) with repeated measures for time indicated a significant main effect for time (p < 0.001) and no significant group x time interaction (p = 0.19). One way ANOVA indicated no differences (p = 0.39) in baseline values.
WOMAC – Pain Going Up and Down Stairs
In comparison to PLA, no statistically significant changes in mean pain relief values when going up and down stairs were identified. Mixed factorial ANOVA (3x15) with repeated measures for time indicated a significant main effect for time (p < 0.001) and no significant group x time interaction (p = 0.59). One way ANOVA indicated no differences (p = 0.24) in baseline values.
Walking Performance
Performance in the 20-meter walk test revealed no statistically significant differences (p > 0.05) between PLA (Pre: 21.3 ± 2.5 seconds vs. Post: 20.0 ± 2.5 seconds; Change: -1.3 ± 3.0 seconds), HOPS1G (Pre: 19.0 ± 3.3 seconds vs. Post: 17.8 ± 3.2 seconds; Change: -1.2 ± 3.9 seconds) and HOPS2G (Pre: 19.6 ± 2.9 seconds vs. Post: 19.7 ± 2.9 seconds; Change: 0.14 ± 2.03 seconds.
Discussion
The primary objective of this study was to explore the potential effects of two different doses of a carbon dioxide extract of hops (Humulus lupulus L.) standardized to 30% alpha acids on joint pain, physical function, and clinical biomarkers in otherwise healthy adults with osteoarthritis of the knee using a randomized, double-blind, placebo-controlled design. Hops contain a spectrum of bioactive ingredients with numerous traditional therapeutic applications. These applications include the treatment of anxiety and insomnia, due to their mild sedative and relaxation effects as well as its use during menopause due to hops’ high phytoestrogen content. Antiinflammatory activity of the alpha acids fraction could potentially help to support the body’s natural response to inflammation and subsequently reduce joint pain. Currently, research is lacking which has sought to identify a dose-response relationship for pain reduction in osteoarthritis patients after hops supplementation. The primary findings from this investigation reveal that two different doses of a hops extract when taken over a 14-day period exhibited (in comparison to placebo) individualized patterns of improvement in pain relief as assessed by questions in the pain subscale of the WOMAC. Functional walking performance (20-meter walk test), however, was not impacted by supplementation.
Hops extracts have been shown, in vitro, to inhibit the production of nitric oxide by suppressing the expression of iNOS (Inducible nitric oxide synthase) [11] and PGE2 [12] while inhibiting COX-2, key pathways in the management of pain caused by inflammation. Selective COX-2 inhibition in mice [14], was confirmed (relative to ibuprofen) in a randomized, double-blind ex vivo design, that standardized hops extract exhibited equivalent COX-2 inhibition but significant COX-1 sparing activity [13]. Our findings revealed a significant reduction in mean perceived pain relief after walking on a flat surface and while in bed at a daily hops extract dose of 1 gram/day (HOPS1G) in individuals with osteoarthritis of the knee. A higher 2-gram dose (HOPS2G) seemed to have no further benefit beyond those identified for HOPS1G. In addition, further effectiveness of hops extract supplementation is supported by the limited use of rescue medication in HOPS1G and HOPS2G in comparison to PLA. Finally, 20-meter walking performance was not impacted by hops extract supplementation. This outcome was not entirely surprising as many factors interact to impact functional performance including strike length, walking ability, motivation, etc. [15]. Both doses were well tolerated with no changes being observed for any of the collected blood biomarkers. Additionally, urinary creatinine and IPF-2 alpha- III levels were not changed throughout the protocol. IPF-2 alpha VI, a marker of oxidative stress, did increase to a greater degree than PLA or HOPS2G. These changes were due largely to HOPS1G having lower levels at baseline and these values returning to levels commensurate with what was measured in the other two groups at day 15.
One critical success factor for nutritional supplementation to improve joint health is a fast reduction of perceived pain to motivate the individual to continuously take the supplement. Hops extract intake, especially at the 2-gram dose, showed a fast-acting effect on mean pain relief with significant improvements occurring in two hours when compared to PLA (Figure 3). Combining fast-acting hops extract with slow-acting dietary supplements commonly used to improve joint health like glucosamine or hydrolyzed collagen that improve function but have small effects on pain [16] might, however, further improve function and quality of life to due increased long-term adherence to supplementation. More research is needed to determine minimal and optimal dose and duration of hops extract supplementation to improve perceived pain and other relevant parameters that impact individuals with various painful and debilitating musculoskeletal conditions.
In terms of safety, hop acids and hop components have been reported in rodent studies [17]. Like the animal work, human studies of different hops extracts have shown no adverse/side effects from the study treatment based on observations of the subject’s complaints by the investigators, or self-reported complaints based on questionnaires [18-20]. The comparison of pre- and postsupplementation blood work and the adverse side effect monitoring in the present study confirmed previous results that hops extract is well-tolerated and will reasonably be expected to be safe under the study’s conditions of use. This study is not without limitations as our supplementation protocol only spanned 14 days and subsequently provides limited insight into longer dosing regimens. In addition, study participants diet was not controlled and they were allowed to use rescue medications throughout which may have confounded the overall outcomes from this project. Similarly, only participants free of previous musculoskeletal disease were recruited into this study trial and consequently, outcomes from this study may hold importance for individuals exhibiting more advanced musculoskeletal joint disease.
Conclusions
In conclusion and in comparison, to PLA, 14-days of supplementing with either a 1-gram or 2-gram dose of hops extract, containing 30% alpha acids, reduced perceived levels of pain as part of the WOMAC assessment without improving walking performance in individuals with osteoarthritis of the knee. Supplementation was well tolerated with no adverse events or safety concerns. Considering the small sample size and limited data available, additional, larger randomized, placebo-controlled trials are needed to confirm these results.
Author Contributions
G.B. conceived the study and participated in design. Writingoriginal draft preparation: M.P., R.J. and C.M.K.; writing-review and editing: B.B. and J.T. All authors read and approved the final manuscript.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Funding
This research was funded by an unrestricted grant from Pharmachem Laboratories LLC, Kearney, NJ, USA. The sponsor played no role in collecting the data, analyzing the data, interpreting the results, or preparing the manuscript.
Acknowledgments
The clinical study was conducted by RTL, Inc., 127 Old Cutter Mill Road, Great Neck, NY 11021, USA. Statistical analysis was conducted by Gary Simon, Ph.D., Professor of Statistics, Stern School of Business, New York University, New York, NY 10003, USA. The authors would like to thank the study participants for their commitment to this protocol.
Conflicts of Interest
B.B. is employed by Ashland LLC which markets Perluxan®. R.J. and M.P. are employed by Increnovo LLC and perform consulting work with Ashland LLC. All other authors declare no competing interests.
References
- Jevsevar DS, Brown GA, Jones DL, Matzkin EG, Manner PA, Mooar P, et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition. The Journal of bone and joint surgery. American volume. 2013; 95: 1885-1886.
- Lee YC. Effect and Treatment of Chronic Pain in Inflammatory Arthritis. Current Rheumatology Reports. 2012; 15: 1-8.
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nature Medicine. 1999; 5: 698-701.
- Martel-Pelletier J, Pelletier J, Fahmi H. Cyclooxygenase-2 and prostaglandins in articular tissues. Seminars in arthritis and rheumatism. 2003; 33: 155-167.
- Brock TG, McNish RW, Peters-Golden M. Arachidonic Acid Is Preferentially Metabolized by Cyclooxygenase-2 to Prostacyclin and Prostaglandin E2 *. The Journal of Biological Chemistry. 1999; 274: 11660-11666.
- Laws D R J. Hop Extracts - A Review. J Institute of Brewing. 1981; 87: 24-29.
- Cleemput MV, Cattoor K, Bosscher KD, Haegeman G, Keukeleire DD, Heyerick A. Hop (Humulus lupulus)-derived bitter acids as multipotent bioactive compounds. Journal of natural products. 2009; 72: 1220-1230.
- Chen W, Becker T, Qian F, Ring J. Beer and beer compounds: physiological effects on skin health. Journal of the European Academy of Dermatology and Venereology. 2014; 28: 142-150.
- Verzele M, Keukeleire DD. Chemistry and Analysis of Hop and Beer Bitter Acids. Amsterdam Netherlands Elsevier. 1991.
- Tagashira M, Watanabe M, Uemitsu N. Antioxidative activity of hop bitter acids and their analogues. Bioscience, biotechnology, and biochemistry. 1995; 59: 740-742.
- Zhao F, Nozawa H, Daikonnya A, Kondo K, Kitanaka S. Inhibitors of nitric oxide production from hops (Humulus lupulus L.). Biological & pharmaceutical bulletin. 2003; 26: 61-65.
- Yamamoto K, Wang J, Yamamoto S, Tobe H. Suppression of cyclooxygenase-2 gene transcription by humulon of beer hop extract studied with reference to glucocorticoid. FEBS Letters. 2000; 465: 103-106.
- Lemay, M., M. A. Murray, A. Davies, H. Roh-Schmidt and K. R. Randolph. In vitro and ex vivo cyclooxygenase inhibition by a hops extract. Asia Pacific journal of clinical nutrition. 2004; 13: S110.
- Hougee S, Faber J, Sanders A, Berg WB, Garssen J, Smit HF, et al. Selective inhibition of COX-2 by a standardized CO2 extract of Humulus lupulus in vitro and its activity in a mouse model of zymosan-induced arthritis. Planta medica. 2006; 72: 228-233.
- Motyl JM, Driban JB, McAdams E, Price LL, McAlindon TE. Test-retest reliability and sensitivity of the 20-meter walk test among patients with knee osteoarthritis. BMC Musculoskeletal Disorders. 2013; 14: 166-166.
- Zhu X, Sang L, Wu D, Rong J, Jiang L. Effectiveness and safety of glucosamine and chondroitin for the treatment of osteoarthritis: a metaanalysis of randomized controlled trials. Journal of Orthopaedic Surgery and Research. 2018; 13.
- Chappel CI, Smith SY, Chagnon M. Subchronic toxicity study of tetrahydroisohumulone and hexahydroisohumulone in the beagle dog. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 1998; 36: 915-922.
- Erkkola R, Vervarcke S, Vansteelandt S, Rompotti P, Keukeleire DD, Heyerick A. A randomized, double-blind, placebo-controlled, cross-over pilot study on the use of a standardized hop extract to alleviate menopausal discomforts. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2010; 17: 389-396.
- Morimoto-Kobayashi Y, Ohara K, Ashigai H, Kanaya T, Koizumi K, Manabe F, et al. Matured hop extract reduces body fat in healthy overweight humans: a randomized, double-blind, placebo-controlled parallel group study. Nutrition Journal. 2015; 15.
- Kyrou I, Christou A, Panagiotakos D, Stefanaki C, Skenderi K, Katsana K, et al. Effects of a hops (Humulus lupulus L.) dry extract supplement on selfreported depression, anxiety and stress levels in apparently healthy young adults: a randomized, placebo-controlled, double-blind, crossover pilot study. Hormones. 2017; 16: 171-180.
- Bellamy N, W W Buchanan, C H Goldsmith, J Campbell, L W Stitt. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988; 15: 1833-1840.
- Milne GL. Classifying oxidative stress by F2-Isoprostane levels in human disease: The re-imagining of a biomarker. Redox Biology. 2017; 12: 897-898.