Research Article
Austin J Reprod Med Infertil. 2014;1(1): 7.
Sperm Sex Ratio (X: Y Ratio) and its Variations
Chaudhury I, Jain M and Halder A*
Department of Reproductive Biology, All India Institute of Medical Sciences, India
*Corresponding author: Halder A, Department of Reproductive Biology, All India Institute of Medical Sciences, New Delhi 110029, India
Received: October 24, 2014; Accepted: November 28, 2014; Published: December 01, 2014
Abstract
Sex ratio can be studied at various levels viz., at the time of spermatogenesis i.e. ratio of X and Y bearing sperm (pre-zygotic sex ratio) or at the time of fertilization/early preimplantation embryo (close to primary sex ratio or post-zygotic sex ratio) or at the time of birth (secondary sex ratio). The natural sex ratio at the time of spermatogenesis is expected to be 1:1. In this study we have examined sex ratio in ejaculated spermatozoa (human) as well as epididymal sperm (mouse) to determine proportion of X and Y bearing sperm i.e., pre-zygotic sex ratio. We also examined effects of seasons (temperature; summer vs. winter), diet (vegetarian vs. non vegetarian), profession (professionals vs. laborer) on sperm (pre-zygotic) sex (X: Y) ratio of ejaculated sperm.
The sperm sex ratio was carried out on 813066 human spermatozoa and 10390 mouse spermatozoa. In human, we have found more (52%) X than Y (48%) bearing sperms (421531X: 391535Y or 1.07X: 1Y). In mouse also we observed preponderance of X (55.5%) as compared to Y (44.5%) bearing sperms (1.24X: 1Y). In all sub-groups (season, diet, profession) we have observed more X bearing sperm. Our observations at pre-zygotic sex ratio have shown skewed sex ratio towards female. A probable reason for this could be preferential elimination of Y bearing sperm. This is also supported by the evidence of more aneuploidy with Y-bearing spermatozoa (~1.5 times more with Y bearing sperms).
Keywords: Spermatozoa; Prezygotic sex ratio (X: Y ratio); Season-Diet- Profession Influence
Introduction
Sex ratio is defined as ratio of number of males to females. Worldwide, the human sex ratio at birth is fairly constant with male excess (51.4%) [1]. The natural sex ratio at the time of spermatogenesis is expected to be 1:1 (accordance with Mendelian segregation principle). Theoretically, offspring sex ratio may be attributed to events that occur before fertilization viz., sperm sex ratio or favor selection of Y or X chromosome bearing spermatozoa or events that occur after fertilization such as preferential survival of embryos of one sex or a combination. Sex ratio in sperm was studied initially by Y-body analysis or analysis of chromosome complements derived from fusion of human sperm with hamster oocytes. With this technique Barry Bean [2] demonstrated an excess of X-bearing sperm. However, Chernos and Martin [3] reported that the sex ratios did not differ significantly on fresh as well as cryopreserved sperm. With the recent advances in molecular technologies sexing of sperm is easily possible thus allowing us to study sperm sex ratio in large numbers of sperms rapidly and made possible to test the hypothesis that a sperm sex ratio underlies a live birth sex ratio bias.
Polymerase chain reaction (PCR) technique for sexing sperm was used initially by many [4,5]. Lobel et al. [4] had found a variation in Y chromosome bearing spermatozoa in humans from 41.9% to 56.7%. Chandler et al. [5] studied the variation in sex ratio in ejaculates in bulls and found that X & Y bearing spermatozoa are unequally distributed per ejaculate in a wave pattern. However with the application of X and Y chromosome FISH to sperm allowed us a more accurate assessment of the sex chromosomal complements [6]. In a sperm fluorescent in situ hybridization (FISH) study by Martin et al. [7] mean frequencies of X and Y bearing sperm was found to be 50.1% and 49.0%, respectively. In a study by Mercier et al. [8] on XYY male, an X: Y ratio of 0.78:1 was found in sperm with normal sex chromosome constitution. Similarly Griffin et al. [9] also reported equal Y and X bearing sperm. However, in contrast Halder and Tutscheck [10] was observed unequal sperm sex ratio, with an excess of X bearing sperm. They also observed higher segregation error of Y chromosome in comparison to X chromosome, thus reducing the number of normal Y bearing sperm further. Similarly, Spriggs et al. [11] identified a small but significant excess of X bearing sperm in a study with 50,000 sperm. Sex ratio distortion is also observed in bovine sperm [12], an excess of X bearing sperm (53%) than Y bearing sperm (46%). However, in a recent [13] Rhesus monkeys study did not find any significant difference between X and Y bearing sperm.
Due to above contradictory reports this study was undertaken to examine sex ratio in ejaculated spermatozoa (human) as well as epididymal sperm (mouse) to determine proportion of X and Y bearing sperm i.e., pre-zygotic sex ratio in a very large number of sperms (over 0.8 millions). We have also examined effects of seasons (temperature), diet & profession on sperm (pre-zygotic) sex ratio.
Material and Methods
Men with normal sperm count [14] were enrolled into the study between February 2008 and March 2011. Study group comprised of 50 subjects (80 samples) among which, 10 subjects were enrolled in season based study (provided semen samples 4 times). In season’s category, semen sample was taken twice in summers (May and August) as well as in winters (December and February). This was done to evaluate the change in sperm sex ratio at the beginning and end of a season and also to find variation in sperm sex ratio according to seasons (temperature). Twenty subjects were included in diet based category; out of them 10 were strict vegetarians and 10 were non-vegetarians (who eat meat, fish etc at least thrice in a week). Another twenty subjects were included for profession based category; out of them 10 were professionals and 10 were daily wage laborer. Age of the volunteers ranged from 21-45 years. Information & biological samples were collected from all cases as per prescribed proforma. In addition we also obtained epididymal sperms from 10 adult male mice.
Institute Animal Ethics Committee & Institute Human Ethics Committee clearance for human & mouse study were obtained (IHEC & IAEC letter nos. T-10/30.01.09 & 485/IAEC/09). Written consent was also obtained from all donors before obtaining semen samples for the study.
Preparation of sperm cells (human) for FISH
Liquefied semen sample was transferred to the 1.5ml micro centrifuge tube and is centrifuged to separate sperm cells and seminal plasma. In the sperm cells pellet 1 ml of phosphate buffer saline (PBS) was added. It was then centrifuged at 8000 rpm for 3mins. The supernatant was removed. This step was repeated 2 more times or when sperm pellet appears clean. Then 1 ml 50mM hypotonic solution (KCl) was added to the sperm pellet, mixed well and incubated at 37°C incubator for 45mins. It was then centrifuged again at 8000rpm for 3mins. About 1 ml fixative (3:1, methanol: acetic acid) was added to the pellet and vortexed and centrifuged at 8000 rpm for 3mins. The supernatant was removed. This procedure was repeated until pellet was whitish and then sperm cells were suspended in fixative and stored at -80°C.
Extraction of mouse spermatozoa
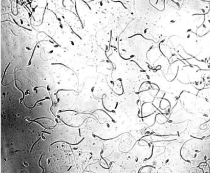
Male mouse (6-8 weeks old) issued from the animal experimental facility, AIIMS. Within 15-20mins of euthanasia epididymis was extracted and placed in petridish containing PBS. Under a zoom dissecting microscope cauda epididymis was gently incised at 2-3 points and left for 3-4mins. This caused release of all live sperms into PBS (Figure 1). PBS along with sperm was collected into 1.5 ml tube & centrifuged at 5000 rpm for 3mins. Supernatant discarded and pellet was given hypotonic treatment for 1 hour. This was followed by 2 washes of fixative and finally stored at -80°C till further use.
Figure 1: Photomicrograph showing mouse sperms.
FISH (human sperm)
FISH was carried out using centromeric probes for chromosome X (red; Cy3 labeled) & Y (green; FITC labeled). Fixed sperm cell suspension was spinned at 5000 rpm for 5 min and supernatant was discarded. Cells were resuspended in a small volume of fresh fixative (3:1 methanol/acetic acid; amount depends on size of the pellet). About 10-15 μl of fixed cell suspension was dropped on the chilled clean glass slide, ensure nuclei spread and left to dry completely. Slide was then flooded for 10 seconds with 3:1 methanol/acetic acid (for fixation of the cell on the slide) and then 70% acetic acid for 1-2 min. Slide was then left to dry and checked under microscope to ensure presence of optimal density of nuclei. Slide was dehydrated in alcohol series (70%, 90% & 100% ethanol) three minutes in each and air dried at room temperature. Then slides (with sperm nuclei attached) were treated with pepsin (1%) in 0.01N HCL for 20 min at 37°C. Slides were then rinsed twice with bi-distilled water and once with PBS. Nuclei on slides then again fixed with 1% Para formaldehyde in PBS for 10 min at 4°C, rinsed twice with PBS and once with bi-distilled water. Slides were then dehydrated in alcohol series (70%, 90% & 100% ethanol) three minutes in each. About 3-5 μl probe mixture (1000 ng labeled probe in 10 μl hybridization buffer containing 60% formamide, 2X SSC, 10% dextran sulfate) were applied on slide containing test sample/cell, cell and probe DNA were denatured together at 76°C for 6 min. and then incubated overnight (18 hours) at 37°C in hybrite chamber.
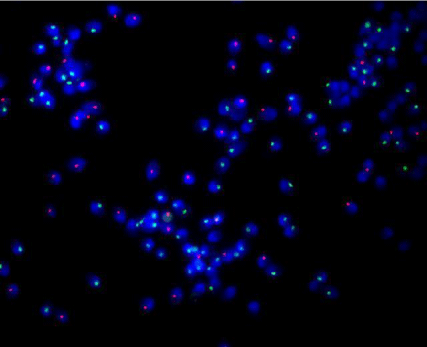
The slides then washed for 2 minutes in 50 ml of solution containing 0.4XSSC/0.3% NP-40 at 72oC water-bath and for 1 minute in 50 ml of solution containing 2XSSC/0.1% NP-40 at room temperature. The slides were dehydrated in descending series of 70%, 90%, and 100% ethanol series 3mins each. Then 10 μl antifade solution (Vector, USA) containing DAPI 1 μg/ml 4´6 diaminidino- 2-phenylindol (DAPI; Sigma USA; counter stain/nuclear stain) applied to the target area onto slide followed by coverslip. Excess of antifade was removed and sealed with nail varnish to avoid moisture. Then slides are viewed using appropriate filter set on Olympus BX 51 fluorescent microscope with a 100 watt mercury bulb using 100X plane appochromatic objective and single band pass filter for DAPI, FITC and TRITC/Cy3 and a dual band pass filter for TRITC and FITC (Olympus Japan). FISH images were captured using FISH Imaging System (Applied Spectral Imaging system & FISH view software version 4.5, Israel). Sperm with red signals were considered as Y bearing sperms and green signals were considered as X-bearing sperms (Figure 2).
Figure 2: XY FISH on human sperms showing either Y (green) or X (red) sperms.
FISH (mouse Sperm)
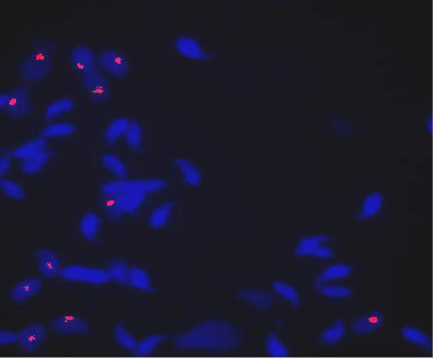
Same as human protocol except slides were incubated in 10mM DTT (Sigma) in a coplin jar for 30mins on ice followed by a dip in milli-Q water at room temperature to decondense sperm nuclei [15] and mouse Y chromosome probe was used. We performed chromosome Y probe-FISH (labeled with Cy3; red) for mouse study. The mouse X probe was excluded from the study due to difficulties in interpretation of results and frequent X probe FISH failure. Sperm with red signals were considered as Y bearing sperm and without any signals were considered as X-bearing sperm (Figure 3).
Figure 3: Chromosome Y FISH on mouse sperm showing Y (red signal) and X (no red signal) bearing sperms.
Results
This study was comprised of 50 subjects (80 semen samples) with normal sperm counts. Cases were divided into three categories as follows:
Seasonal study (temperature effects)
Winter season: samples collected twice in the month of December (beginning of winter) & February (end of winter) on 10 subjects (20 samples).
Summer season: samples collected twice in the month of May (beginning of summer) & August (end of summer) on same 10 subjects (20 samples).
Diet study
There were 10 subjects each from vegetarian diet group (10 samples) and non-vegetarian diet group (10 samples).
Profession
There were 10 subjects each from professional groups (doctor/ engineer; 10 samples) and laborers (daily wage laborer; 10 samples).
Sex chromosome specific (XY) Fluorescent In-Situ Hybridization (FISH) was carried out in all cases using centromeric chromosome X and Y probes (Figure 2). About 10,000 sperm nuclei were counted in each semen samples and X/Y ratio (sex ratio) was calculated. Overall sex ratio was observed as 1.076:1. Tables 1-7 are showing details of FISH results in various groups.
SN
Dec X
Dec Y
Total sperm
Dec X/Y
Feb X
Feb Y
Total sperm
Feb X/Y
1
5318
5090
10408
1.045
5125
4924
10049
1.041
2
5672
5844
11516
0.971
5127
4936
10063
1.039
3
5374
4881
10255
1.101
5259
4995
10254
1.053
4
5216
5218
10434
1
5215
4922
10137
1.06
5
5378
4920
10298
1.093
5184
4862
10046
1.066
6
5008
5008
10016
1
5175
4886
10061
1.059
7
5223
4827
10050
1.082
5386
5128
10514
1.05
8
5226
4799
10025
1.089
5214
4901
10115
1.064
9
5248
4812
10060
1.091
5411
4670
10081
1.159
10
5418
4700
10118
1.153
5314
4802
10116
1.107
Total
53081
50099
103180
1.06
52410
49026
101436
1.069
Table 1.1: Sex Ratio in beginning (December) and end (February) of winter season.
Month
X bearing sperm
Median (min-max)
Y bearing sperm
Median (min-max)
p-value
X/Y ratio
Median (min-max)
Dec
5283 (5008-5672)
4900.5 (4700-5844)
0.0072
1.086 (0.97-1.15)
Feb
5214 (5125 - 5411)
4911.5 (4670-5128)
0.0084
1.06 (1.039-1.159)
p-value
0.1736
0.879
0.9397
Table 1.2: Statistical significance of Sperm FISH data for winter (n=10)
SN
May X
May Y
Total sperm
May X/Y
Aug X
Aug Y
Total sperm
Aug X/Y
1
5313
4953
10266
1.073
5471
4699
10170
1.164
2
5296
4890
10186
1.083
5314
5222
10536
1.018
3
5229
4831
10060
1.082
5030
4999
10029
1.006
4
5231
4774
10005
1.096
5121
4901
10022
1.045
5
5193
5041
10234
1.03
4982
5070
10052
0.983
6
5282
4847
10129
1.09
5440
4862
10302
1.119
7
5383
4824
10207
1.116
5143
4897
10040
1.05
8
5446
4862
10308
1.12
5222
4816
10038
1.084
9
5334
4800
10134
1.111
5223
4999
10222
1.045
10
5439
4632
10071
1.174
5190
4899
10089
1.059
Total
53146
48454
101600
1.097
52136
49364
101500
1.056
Table 2.1: Sex Ratio in the beginning (May) and end (August) of summer season.
Month
X bearing sperm
Median (min-max)
Y bearing sperm Median (min-max)
p-value
X/Y ratio
Median (min-max)
May
5304.5
(5193-5446)
4839
(4632-5041)
0.0051
1.093
(1.03-1.174)
Aug
5206
(4982-5471)
4900
(4699-5222)
0.0093
1.0475
(0.983-1.164)
p-value
0.0821
0.0887
0.0587
Table 2.2: Statistical significance of Sperm FISH data for summer (n=10)
Season
X bearing sperm
Median (min-max)
Y bearing sperm Median(min-max)
p-value
X/Y ratio
Median (min-max)
X/X+Y
Median (min-max)
Winter
10585.5
(10183-10799)
9885
(9482-10780)
0.0051
1.071
(1.002-1.129)
0.517
(0.5-0.53)
Summer
10583.5
(10175-10784)
9693.5
(9531-10112)
0.0051
1.0825
(1.006-1.117)
0.52
(0.502-0.528)
p-value
0.8206
0.3256
0.4270
0.3841
Table 2.3: Statistical significance of Sperm FISH data for total winters and summers (n=20)
Samples
X bearing sperm
Y bearing sperm
Total sperm
X/Y ratio
1
5375 (53.3%)
4709 (46.7%)
10084
1.14
2
5591 (53.4%)
4887 (46.6%)
10478
1.14
3
5221 (52%)
4817 (48%)
10038
1.08
4
5246 (51.8%)
4873 (48.2%)
10119
1.08
5
5284 (52.4%)
4794 (47.6%)
10078
1.10
6
5437 (52.1%)
4989 (47.9%)
10426
1.09
7
5237 (52.1%)
4822 (47.9%)
10059
1.09
8
5137 (51.2%)
4898 (48.8%)
10035
1.05
9
5222 (51%)
5010 (49%)
10232
1.04
10
5331 (52.5%)
4827 (47.5%)
10158
1.10
Total
53081 (52.19%)
48626 (47.81%)
101707
1.09
Table 3: Sperm Sex Ratio calculated for Vegetarian diet.
Samples
X bearing sperm
Y bearing sperm
Total sperm
X/Y ratio
1
5290 (52.5%)
4794 (47.5%)
10084
1.10
2
5429 (51.5%)
5107 (48.5%)
10536
1.06
3
5171 (51.4%)
4887 (48.6%)
10058
1.06
4
5262 (52.5%)
4759 (47.5%)
10021
1.11
5
5200 (51.2%)
4948 (48.8%)
10148
1.05
6
5123 (51.2%)
4888 (48.8%)
10011
1.05
7
4998 (49.9%)
5010 (50.1%)
10008
1.00
8
5211 (51.5%)
4899 (48.5%)
10110
1.06
9
5335 (52.5%)
4822 (47.5%)
10157
1.11
10
5246 (51.8%)
4888 (48.2%)
10134
1.07
Total
52265 (51.6%)
49002 (48.4%)
101267
1.066
Table 4.1: Sperm Sex Ratio calculated for Non-Vegetarian diet.
Diet type
X bearing sperm
Median (min-max)
Y bearing sperm Median (min-max)
p-value
X/Y ratio
Median (min-max)
Vegetarian
5265 (5137-5591)
4850 (4709-5010)
0.0051
1.088 (1.042-1.144)
Non- Vegetarian
5228.5 (4998-5429)
4888 (4759-5107)
0.0069
1.0635 (0.998-1.106)
p-value
0.2121
0.3634
0.2263
Table 4.2: Statistical significance of Sperm FISH data for diet (n=20)
Professional
X bearing sperm
Y bearing sperm
Total sperm
X/Y ratio
1
5130 (51%)
4921 (49%)
10051
1.04
2
5231 (51.6%)
4911(48.4%)
10142
1.07
3
5111 (50.9%)
4923 (49.1%)
10034
1.04
4
5176 (51.5%)
4880 (48.5%)
10056
1.06
5
5335 (52.5%)
4834 (47.5%)
10169
1.10
6
5246 (51.7%)
4901 (48.3%)
10147
1.07
7
5375 (53.3%)
4708 (46.7%)
10083
1.14
8
5437 (53.6%)
4700 (46.4%)
10137
1.16
9
5591 (54.3%)
4702 (45.6%)
10293
1.19
10
5223 (52.1%)
4811 (47.9%)
10034
1.09
Total
52855 (52.26%)
48291 (47.74%)
101146
1.09
Table 5: Sperm Sex Ratio calculated for Professions.
Laborers
X bearing sperm
Y bearing sperm
Total sperm
X/Y ratio
1
5339 (52.2%)
4894 (47.8%)
10233
1.09
2
5211 (51.6%)
4882 (48.4%)
10093
1.07
3
5340 (53.3%)
4680 (46.7%)
10020
1.14
4
5010 (50.1%)
4998 (49.9%)
10008
1.00
5
5200 (51.2%)
4950 (48.8%)
10150
1.05
6
5171 (51.6%)
4859 (48.4%)
10030
1.06
7
5284 (52.3%)
4822 (47.7%)
10106
1.10
8
5331 (52.5%)
4823 (47.5%)
10154
1.11
9
5234 (52.1%)
4820 (47.9%)
10054
1.09
10
5437 (52.2%)
4988 (47.8%)
10425
1.09
Total
52557 (51.8%)
48716 (48.2%)
101273
1.08
Table 6.1: Sperm Sex Ratio calculated for Laborers.
Professions
X bearing sperm
Median (min-max)
Y bearing sperm Median (min-max)
p-value
X/Y ratio
Median (min-max)
Professionals
5239 (5111-5591)
4857 (4700-4923)
0.0051
1.078 (1.038-1.189)
Laborers
5259 (5010-5437)
4871 (4680-4998)
0.0051
1.088 (1.002-1.141)
p-value
0.8501
0.4963
0.7913
Table 6.2: Statistical significance of Sperm FISH data for Profession (n=20)
Total X bearing sperm (Mean + SE)
Total Y bearing sperm (Mean + SE)
p-value
X/Y ratio
421531 (7025.517 + 323.980
391535 (6525.583 + 306.508)
0.0001
1.076:1
Table 7: Showing statistical significance of total human sperm data analysis (n=80).
We observed that the number of X bearing sperms were significantly more than the number of Y bearing sperm in all groups and irrespective of subgroups viz., winters or summer (p=0.0051). We did not find any statistically significant difference in median values of X/Y ratio between summer and winter (p=0.4270). However, we observed more X bearing sperm in winter, although statistically insignificant (p=0.8206).
The number of X bearing sperm were found to be significantly more than the number of Y bearing sperm in both vegetarian (p=0.0051) and non-vegetarian diet group (p=0.0069). We observed more X bearing sperm in vegetarian than non-vegetarian diet group (p= 0.2121), but statistically not significant.
The number of X bearing sperm also were found to be significantly more than the number of Y bearing sperm in professional group (p=0.0051) as well as in laborer group (p=0.0051). However difference in median values of X/Y ratio between two subgroups were statistically insignificant (p=0.7913).
In mouse FISH study also we observed significantly more (p=0.0051) X bearing sperms than the number of Y bearing sperms (Table 8).
Mice
X bearing sperm
(sperm without Y signal)
No. of Y bearing sperm (sperm with Y signal)
Total sperm
X/Y
1
562 (55.4%)
452 (44.6%)
1014
1.24
2
589 (55.3%)
476 (44.7%)
1065
1.24
3
594 (56%)
466 (44%)
1060
1.27
4
581 (55.8%)
460 (44.2%)
1041
1.26
5
587 (56.6%)
451 (43.4%)
1038
1.3
6
578 (56.3%)
449 (43.7%)
1027
1.29
7
567 (55.75%)
450 (44.25%)
1017
1.26
8
545 (53.1%)
481 (46.9%)
1026
1.133
9
569 (54.4%)
476 (45.6%)
1045
1.19
10
590 (55.8%)
467 (44.2%)
1057
1.26
Total
5762 (55.45%)
4628 (44.55%)
10390
1.24
Table 8.1: Y-Fish Result on 10 Adult Male Mice Sperm Samples.
X bearing sperm
Median (min-max)
Y bearing sperm Median (min-max)
p-value
X/Y ratio Median (min-max)
579.5 (545-594)
463 (449-481)
0.0051
1.26 (1.133-1.3)
Table 8.2: Statistical significance of Sperm FISH data for mice (n=10)
Discussion
The study on the pre-zygotic/sperm sex ratio was carried out on 813066 human spermatozoa and 10390 mouse spermatozoa. At the pre-zygotic level in human study, we have found more (52%) X than Y (48%) bearing sperms (421531X: 391535Y or 1.07X:1Y). We have also observed more X in almost all samples (76/80) irrespective of subgroups viz., season, diet or profession. In mouse (epididymal sperms) also we observed preponderance of X (55.5%) as compared to Y (44.5%) bearing sperms (1.24X:1Y). We have also taken into account the effect of various environmental factors like temperature, diet, profession on human sperm sex ratio. In all sub-groups as well as in mouse we have observed more X bearing sperm. A probable reason for this could be presence of meiotic drive elements which could lead to incapacitation or fragmentation of Y bearing sperm or preferential elimination of Y bearing sperm through apoptosis mechanism. This is also supported by the evidence of more aneuploidy with Y-bearing spermatozoa (~1.5X more with Y bearing sperms; 192 aneuploid Y sperms and 124 aneuploid X sperms). This is also we observed in our previous study [10]. This indicates more X bearing sperms available for fertilization.
We have investigated influence of season (temperature) on sperm sex ratio. Seasonal (temperature) variation in sex ratio has been observed in variety of vertebrates viz. frog, fishes, lizard, birds, reptiles, etc [16-18]. In fishes it was shown that male population increases on increase in temperature of water. In human also temperature based variation in secondary sex ratio was observed [19]. According to the findings of Cagnacci et al. [20] sex ratio at the time of conception showed a seasonal rhythm, higher in 3 months of peak (September, October, November) than in 3 months of nadir (March, April, May). We wanted to check whether such skewing originates at sperm level, so we carried out the sperm sex ratio study in two different time points viz. winter and summer. In winters, we also tried to find out the change in sperm sex ratio at beginning (December) as well as at end (February) of each seasons. Similarly, we investigated the sperm sex ratio in beginning (May) and end (August) of summer. Approximately 10,000 sperm were counted per individual after performing XY-FISH on sperm nuclei. We observed more X bearing sperm (210773) than Y bearing sperm (196900) in both winters and summers (more so at beginning i.e. December: 53081 & May: 53146 than at the end i.e. February: 52410 & August: 52136). However, sex ratio (X/Y) change at the beginning and end of winter as well as summer was statistically insignificant. The X/Y ratio in December, February, May, August was 1.06:1, 1.069:1, 1.097:1, 1.056:1 respectively; which is just reverse of secondary sex ratio (sex ratio at birth) i.e., male:female as 1.07:1. When the two seasons i.e. winters and summers as a whole were considered then also our data showed statistically insignificant, however X bearing sperm were significantly more than Y bearing sperm. Thereby we can say that there seems to be no difference in sperm sex ratio upon seasons.
We have also investigated influence of diet with sperm sex ratio. The origin of the study was the study of Lloyd et al. [21] who reported that butchers (who themselves eat more non-vegetarian food) bore more sons. We speculated that the skew originated at the spermatozoa level. However we found significantly more X bearing sperm than Y bearing sperm in all groups. Mean X/Y ratio for vegetarian category was 1.09:1 and for non-vegetarian category was 1.067:1. Inter-categorically X/Y ratio didn’t differ in spite the fact that there were more X bearing sperm in the vegetarian category. We observed that diet (vegetarian or non-vegetarian) do not seem to cause an effect on sperm sex ratio.
Our third group for sperm sex ratio was with profession i.e., professionals vs. laborers. We selected these two groups, as there are several studies reported which seem to show an association of paternal profession and birth sex ratio [22-25]. Literature suggests that work place exposure (to different chemicals) can cause a shift in sperm sex ratio [26-28]. We selected two extreme groups, one who do more physical work and the other who are indulged in mental work like educated professionals (scientist, doctor, etc). We observed more X bearing sperm than Y bearing sperm in both categories. Mean value of X/Y ratio in professionals and laborers was 1.09 and 1.08 respectively. We couldn’t find statistically significant difference in 2 groups.
In all the three study subgroups i.e. seasons, diet and profession we observed significantly more X bearing sperm (210773, 105346 and 105412 respectively) than Y (196900, 97628 and 97007 respectively) bearing sperms. In totality of human sperm data, X:Y ratio was 1.076:1 (or can be represented as Y:X :: 0.929:1). Thus it seems unlikely that variation in gametic sex ratio is an important contributor to excess of males (1.07 male: 1female) observed at birth. Our results are supported by various other studies [7,11] as well as contradicted by some [8] who have observed more Y bearing sperm whereas Griffin et al. [9] found equal ratio for X and Y bearing sperms.
During our study we have also observed Y bearing sperms with more sex chromosome aneuploidy (192 aneuploid Y sperms and 124 aneuploid X sperms i.e., 1.5 times more). More aneuploidy in Y bearing sperm was also observed in some other study [10] in which they observed higher segregation error with Y chromosome thus further reducing normal Y bearing sperm concentration. This work was extended on mouse epididymal sperm. Our mouse data also suggest a surfeit of X bearing sperm (5762 i.e. 55.5%) as compared to Y bearing sperm (4628 i.e. 44.5%).
This study finds excess of X bearing sperms both in human and mouse. This outcome cannot be considered as chance as interpretation was derived from study of over 0.8 millions of sperms. A probable reason for this could be presence of meiotic drive elements which could lead to incapacitation or fragmentation of Y bearing sperm or preferential elimination of Y bearing sperm through apoptosis mechanism. Stalked eye fliers often have an extreme sex ratio due to meiotic drive element on the X chromosome [Xd]. Male carriers of [Xd] show a decrease of meiotic drive intensity and represented as resistant Y chromosome [Ym]. Resistant Y chromosome [Ym] when paired with [Xd] cause the transmission of predominantly Y sperm [29]. Similarly, in fruit fly an X linked meiotic drive has been reported. Males that are carriers of X linked drive produce an abundance of female offspring caused by a deficiency of Y bearing sperm. Non-disjunction of Y chromatids during meiosis II results in failure of non disjunctioned (YY) spermatids to develop into functional sperm [30]. High sex ratio distortion has also been shown to run in families [12]. Szyda et al. [12] looked for recombination rate of pseudoautosomal region of the sex chromosome and found a significant skew in the X and Y chromosomes. He proposed two hypotheses for this deviation. The most straight forward hypothesis was that recombination can produce a certain combination of alleles that are detrimental to Y – sperm viability or preferential to X-sperm viability. Y recombinant products were being lost at some stage during spermatogenesis. The alternative hypothesis was that there may be X specific genes located near the bovine pseudoautosomal region, that when expressed affect the viability of the Y bearing sperm. Similarly, in the mouse, deletions on the Y-chromosome long arms (MSYq) leads to the up- regulation of multiple X- and Y-linked transcripts in spermatids. Two suspected genes are the X-linked multi-copy gene Xmr and its counterpart MSYq-linked Sly, which are up- and down-regulated, respectively, in the testes of MSYqdel males [31] leading to distortion of sex ratio.
Conclusion
Our results at Pre-zygotic/sperm level study have shown skewed sex ratio towards X/female. In contrast to pre-zygotic/sperm sex ratio, secondary sex ratio (sex ratio at birth) all over the world shows an excess of males. Hence, we conclude that the mechanisms that cause skewing of secondary sex ratio is not through altered sperm/ prezygotic sex ratio. We also conclude that excess of X bearing sperm in spermatogenesis is real (almost all samples i.e., 76/80 have similar findings) and thus have biological basis.
References
- James WH. Further evidence that mammalian sex ratios at birth are partially controlled by parental hormone levels around the time of conception. Hum Reprod. 2004; 19: 1250-1256.
- Bean B. Progenitive sex ratio among functioning sperm cells. Am J Hum Genet. 1990; 47: 351-353.
- Chernos JE, Martin RH. A cytogenetic investigation of the effects of cryopreservation on human sperm. Am J Hum Genet. 1989; 45: 766-777.
- Lobel SM, Pomponio RJ, Mutter GL. The sex ratio of normal and manipulated human sperm quantitated by the polymerase chain reaction. Fertil Steril. 1993; 59: 387-392.
- Chandler JE, Steinholt-Chenevert HC, Adkinson RW, Moser EB. Sex ratio variation between ejaculates within sire evaluated by polymerase chain reaction, calving, and farrowing records. Journal of Dairy Science. 1998; 81: 1855-1867.
- Goldman AS, Fomina Z, Knights PA, Hill CJ, Walker AP, Hultén MA. Analysis of the primary sex ratio, sex chromosome aneuploidy and diploidy in human sperm using dual-colour fluorescence in situ hybridization. Eur J Hum Genet. 1993; 1: 325-334.
- Martin RH, Spriggs E, Rademaker AW. Multicolor fluorescence in situ hybridization analysis of aneuploidy and diploidy frequencies in 225,846 sperm from 10 normal men. Biol Reprod. 1996; 54: 394-398.
- Mercier S, Morel F, Roux C, Clavequin MC, Bresson JL. Analysis of the sex chromosomal equipment in spermatozoa of a 47,XYY male using two-colour fluorescence in-situ hybridization. Molecular Human Reproduction. 1996; 2: 485-488.
- Griffin DK, Abruzzo MA, Millie EA, Feingold E, Hassold TJ. Sex ratio in normal and disomic sperm: evidence that the extra chromosome 21 preferentially segregates with the Y chromosome. Am J Hum Genet. 1996; 59: 1108-1113.
- Halder A, Tutscheck B. Analysis of meiotic segregation in human nondecondensed interphase spermatozoa by triple colour rapid direct fluorescent in situ hybridization. Indian Journal of Medical Research. 1998; 107: 94-97.
- Spriggs EL, Rademaker AW, Martin RH. Aneuploidy in human sperm: the use of multicolor FISH to test various theories of nondisjunction. Am J Hum Genet. 1996; 58: 356-362.
- Szyda J, Simianer H, Lien S. Sex ratio distortion in bovine sperm correlates to recombination in the pseudoautosomal region. Genet Res. 2000; 75: 53-59.
- Hung PH, Froenicke L, Lyons LA, VandeVoort CA. Nothing 'FISH'y about the rhesus macaque sex ratio. J Med Primatol. 2009; 38: 42-50.
- WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4th Edition. ISBN: 9780521645997. Copublisher: The World Health Organization 1999.
- Hill FS, Marchetti F, Liechty M, Bishop J, Hozier J, Wyrobek AJ. A new FISH assay to simultaneously detect structural and numerical chromosomal abnormalities in mouse sperm. Mol Reprod Dev. 2003; 66: 172-180.
- Matsuba C, Miura I, Merilä J. Disentangling genetic vs. environmental causes of sex determination in the common frog, Rana temporaria. BMC Genet. 2008; 9: 3.
- Ospina-Alvarez N, Piferrer F. Temperature-dependent sex determination in fish revisited: prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS One. 2008; 3: e2837.
- Rhen T, Schroeder A, Sakata JT, Huang V, Crews D. Segregating variation for temperature-dependent sex determination in a lizard. Heredity (Edinb). 2011; 106: 649-660.
- McLachlan JC, Storey H. Hot male: can sex in humans be modified by temperature? J Theor Biol. 2003; 222: 71-72.
- Cagnacci A, Renzi A, Arangino S, Alessandrini C, Volpe A. The male disadvantage and the seasonal rhythm of sex ratio at the time of conception. Hum Reprod. 2003; 18: 885-887.
- Lloyd MM, Lyster WR, Lloyd OL. Birth sex ratios and prostatic cancer in butchers. Lancet. 1987; 1: 561.
- Snyder RG. The sex ratio of offspring of pilots of high performance military aircraft. Hum Biol. 1961; 33: 1-10.
- Zadeh HG, Briggs TW. Ionising radiation: are orthopaedic surgeons' offspring at risk? Ann R Coll Surg Engl. 1997; 79: 214-220.
- Bernstein ME. Evidence of genetic variation of the primary sex ratio in man. Journal of Heredity. 1954; 45: 59-64.
- Bernstein ME. Sex distribution in sibships according to occupation of parents. Genus. 1981; 37: 179-188.
- Robbins WA, Wei F, Elashoff DA, Wu G, Xun L, Jia J. Y:X sperm ratio in boron-exposed men. J Androl. 2008; 29: 115-121.
- Tiido T, Rignell-Hydbom A, Jonsson BA, Giwercman YL, Pederson HS, Wojtyniak B, et al. Impact of PCB and p.p’-DDE contaminants on sperm Y:X chromosome ratio: studies in three European populations and the inuit population in Greenland. Environmental Health Perspectives. 2006; 114: 718-724.
- Tiido T, Rignell-Hydbom A, Jönsson BA, Rylander L, Giwercman A, Giwercman YL. Modifying effect of the AR gene trinucleotide repeats and SNPs in the AHR and AHRR genes on the association between persistent organohalogen pollutant exposure and human sperm Y:X ratio. Mol Hum Reprod. 2007; 13: 223-229.
- Presgraves DC, Severance E, Wilkinson GS. Sex chromosome meiotic drive in stalk-eyed flies. Genetics. 1997; 147: 1169-1180.
- Cazemajor M, Joly D, Montchamp-Moreau C. Sex-ratio meiotic drive in Drosophila simulans is related to equational nondisjunction of the Y chromosome. Genetics. 2000; 154: 229-236.
- Ellis PJ, Clemente EJ, Ball P, Touré A, Ferguson L, Turner JM, et al. Deletions on mouse Yq lead to upregulation of multiple X- and Y-linked transcripts in spermatids. Hum Mol Genet. 2005; 14: 2705-2715.