
Review Article
J Stem Cell Res Transplant. 2017; 4(1): 1026.
Dissecting the Role of Sox2 in Stemness Regulation and Regenerative Medicine
Chanoumidou K1,2, Hadjimichael C¹, Vogiatzoglou A1,3 and Kretsovali A1*
¹Institute of Molecular Biology and Biotechnology, Foundation for Research and Technology – Hellas (FORTH), 70013 Heraklion, Crete, Greece
²Department of Molecular Biology and Genetics, Democritus University of Thrace, Dragana, Alexandroupolis, 68100 Evros, Greece
³Department of Biology, University of Crete, 71409 Heraklion, Crete, Greece
*Corresponding author: Androniki Kretsovali, Institute of Molecular Biology and Biotechnology, Foundation for Research and Technology – Hellas (FORTH), 70013 Heraklion, Crete, Greece
Received: October 18, 2016; Accepted: January 20, 2017; Published: January 23, 2017
Abstract
The necessity of Sox2 for successful establishment of pluripotent state and maintenance of adult stem cells highlights its role in stemness regulation during development. Interestingly, Sox2 is implicated in both self-renewal maintenance and differentiation decisions. Its role in pluripotent cells is well documented whereas recent studies underline Sox2 importance in neural and mesenchymal stem cells function. Reprogramming experiments have revealed the potential of Sox2 for imposing changes in cell fate. In particular, Sox2 is essential for induced pluripotent cells generation and somatic cells conversion into another type. Sox2 pro-neural role has been utilized for the somatic cells and mesenchymal stem cells transition to induced neural stem cells as well as for their trans-differentiation to induced Neurons. This conversion ability, in combination with its role as pro-sensory factor, nominates Sox2 a key player in transplantation therapies. In this review, we discuss the multiple roles of Sox2 in the regulation of pluripotent, neural and mesenchymal stem cells pointing to their applications for tissue regeneration.
Keywords: Sox2; Pluripotency; Stem cells; Differentiation; Regenerative medicine
Abbreviations
ESC: Embryonic Stem Cells; NSC: Neural Stem Cells; MSC: Mesenchymal Stem Cells; iPSC: induced Pluripotent Stem Cells; NPC: Neural Progenitor Cells; iNSC: Induced Neural Stem Cells; iN: induced Neurons; CNS: Central Nervous System; SVZ: Subventricular Zone; DG: Dentate Gyrus; ENS: Enteric Nervous System
Introduction
Sex determining region Y-box 2 (Sox2) belongs to the SOX family that consists of transcription factors with a single High Mobility Group (HMG) box DNA– binding domain [1]. To date, twenty Sox genes have been discovered in mammals that are further divided into subgroups from A to H, based on the homology degree in the HMG domain [2]. Sox2 is the most representative and well-studied member of SoxB1 group, which also includes Sox1 and Sox3 [1].
Functionally, Sox2 is crucial for successful embryonic development whereas its abnormal activity has been connected to cancer development [3]. Sox2 is involved in cancer physiology through several mechanisms that vary depending on the cancer type. Particularly, Sox2 overexpression influences cell proliferation, invasion, apoptosis and metastasis via regulating oncogenic pathways, including Wnt/β-catenin, PI3K/mTOR, JAK/STAT3 and EGFR signaling [3]. Many studies investigate its function in embryonic and adult stem cells highlighting its role as both stemness factor, as well as lineage specifier. Mediators of Sox2 regulatory network include many transcription factors, microRNAs as well as epigenetic and signaling pathways regulators [4]. In this review, we discuss the aforementioned biological roles of Sox2, with particular emphasis in embryonic, neural and mesenchymal stem cells regulation.
The Multifunctional Role of Sox2 in Stemness Regulation
Sox2 function in Embryonic Stem Cells
Sox2 expression is detected in the Inner Cell Mass (ICM) and extra embryonic ectoderm of pre-implantation blastocysts [5]. Its deletion in the zygote, results in early embryonic lethality due to failure of epiblast formation with no impact on trophectoderm development [5]. Owing to Sox2 deficient mice lethality [5], analysis of hypomorphic mice mutants were necessary for the investigation of Sox2 functional role during embryonic development. Using the aforementioned mutants with decreased expression of Sox2, Que and colleagues illustrated that Sox2 plays important role in endoderm development. Specifically, Sox2 is involved in the differentiation and morphogenesis of esophagus, trachea and lung, while its reduction leads to the abnormal development of lung and esophageal atresia as well as tracheal-esophageal fistula defects [6]. Additionally, Sox2 is involved in the development of ectoderm that will be discussed later on. Interestingly, heterozygous Sox2 mice are phenotypically normal, although the pituitary size, hormone production and testicular size are reduced [7]. Hence, Sox2 seems to be a central regulator for early Pluripotent Stem Cells (PSC) formation and embryonic development.
In accordance with the data in pre-implantation embryos, Sox2 is highly expressed in Embryonic Stem Cells (ESC), where together with the proteins Oct4 and Nanog constitute the core transcriptional network responsible for stemness maintenance. Strikingly, a synergistic function of Sox2 and Oct4 for the activation of Oct-Sox enhancers/promoters has been identified, leading to the regulation of various transcription factors [5]. In particular, they activate the expression of pluripotent genes (Nanog, Sox2, Oct4 etc), while suppressing the expression of key genes essential for the in vitro differentiation and in vivo developmental processes (Pax6, Gbx2) [8,9]. Although Sox2 has a pivotal role in gene expression regulation, it is striking to find that Oct-Sox enhancers are still activated in Sox2/- ESC. This suggests that Oct-Sox complexes could be also formed by a direct interaction between Oct4 and other Sox family members [10]. Interestingly, forced expression of Oct4 partially rescues the phenotype of Sox2 loss of function [11], proving that Sox2 is critical for the maintenance of stem cell identity mainly through securing Oct4 expression levels. To conclude, Sox2-Oct4 interaction and their (auto)-regulatory activity is of paramount importance for ESC selfrenewal and pluripotency maintenance [10,12].
Except for its function as a stemness factor in PSC, Sox2 also orchestrates the cell fate decision. Sox2 expression levels need to be strictly optimized in ESC, whilst either higher or lower levels disrupt ESC self-renewal and promote their differentiation [11,13]. More specifically, reduction of Sox2 expression drives cells towards trophectoderm, whereas conflicting are the results considering the effects of Sox2 overexpression. Kopp and colleagues observed that in mouse ESC (mESC) forced expression of Sox2 more than four folds caused massive cell death, while small increases of its expression level lead to exit from pluripotency and differentiation towards all neuroectoderm, mesoderm and trophectoderm [13]. On the contrary, another study reported that overexpression of Sox2 does not impair mESC self-renewal but biases lineage choice in favor of neuroectoderm only under serum-free culture conditions (Figure 1) [14].
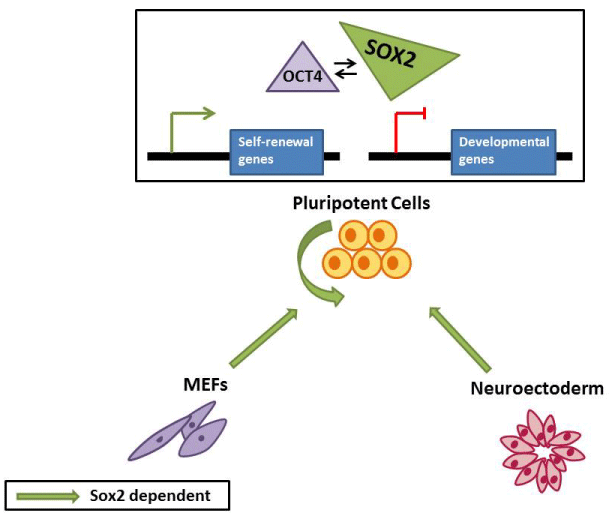
Figure 1: Sox2 functions in PSC. Sox2 participates in the reprogramming
of somatic cells to induced PSC and promotes their self-renewal through
transcriptional regulation of target genes in conjunction with Oct4
transcription factor. Additionally, Sox2 governs PSC commitment towards
neuroectodermal lineage.
Sox2 in reprogramming of somatic cells to induced PSCs
In view of the fact that Sox2 is crucial in ESC establishment and maintenance, it is possible that its ectopic expression in somatic cell types is sufficient to change cell fates. In 2006, Yamanaka and Takahashi established a revolutionary technology in nuclear reprogramming field. They uncovered a set of transcription factors -Oct4, Sox2, Klf4, c-Myc (OSKM)- that can reprogram somatic cells to pluripotent state [15]. Several studies proposed that reprogramming requires a stepwise transition through key sequential events in order to be successful. Sox2 is essential in the last phase of reprogramming process [9] and its activation causes deterministic events (Figure 1). In particular, Sox2 activates its own transcription as well as others of pluripotency-associated genes to stabilize the pluripotent state and finally to generate an induced PSC (iPSC) [16]. Jaenisch and co workers proposed that the endogenous Sox2 locus activation throughout reprogramming process leads to several transcriptional changes in cells intended to form iPSC [17]. Moreover, it has been suggested that Sox2 can be replaced by only two of the Sox family members –Sox1 and Sox3 - due to their ability to interact with Oct4 and regulate the expression of the target genes [18]. Consequently, Sox2 is dispensable for pluripotency gene activation in somatic cells following cell fusion with ESC.
Sox2 as a master regulator of Neural Stem Cells
From zebrafish to human, the conserved expression profile of Sox2 during development and adulthood suggests its involvement at the establishment and proper function of the Central Nervous System (CNS) [19,20]. Expression of Sox2 is detected already at the morula stage of the embryo and becomes restricted mainly to neuroectoderm during gastrulation [5]. Subsequently, it is predominantly expressed in embryonic Neural Stem Cells (NSC) as well as in adult NSC and Neural Progenitor Cells (NPC) at the postnatal neurogenic regions of the Sub Ventricular Zone (SVZ) and Hippocampus Dentate Gyrus (DG) [21]. Sox2 expression persists until the precursor cells differentiate, however it has been also found in certain mature neuronal cells and astrocytes [21,22]. Thus, despite the previously analyzed role of Sox2 in neural commitment of pluripotent cells, it is also associated with the maintenance of NSC properties [23]. NSC are multipotent cells of the nervous system, defined by the ability to differentiate and give rise to neurons and glial cells within the clonal progeny of a single stem cell [24]. The fact that Sox2 is able to reprogram alone somatic cells to induced NSC further demonstrates the essential function of Sox2 in these cells [25,26].
Gain and loss of function experiments highlight the vital role of Sox2 in the developing CNS and neurogenesis. Forced expression of Sox2 inhibits neuronal differentiation of NPC, whereas Sox2 downregulation facilitates it, promoting exit from cell cycle [27,28]. Due to Sox2 requirement in epiblast establishment during development, mice with complete depletion of Sox2 fail to survive after implantation. As a result, loss of function experiments have been performed using conditional null mutations or hypomorphic ones, by targeting gene regulatory regions.
There is a range of observing phenotypes depending on the kind of mutation and the developmental stage at which they are established. Conditional pan-neural deletion of Sox2 led to negligible brain defects and mice lethality at birth [29]. However, Ferri and colleagues showed that Sox2 mutants present brain defects including reduced cortex volume and epilepsy [21]. At the cellular level, reduction of GFAP-positive NSC in the hippocampus DG, and of neurogenesis in both DG and SVZ, was reported. In agreement, in another study, conditional neural specific deletion of Sox2 at E12.5 resulted in mice that died a few weeks after birth [25]. In P7 neurogenesis was entirely lost in the DG of hippocampus interfering with its development and subsequently leading to DG hypoplasia. The different level of consequences in the two neurogenic regions hints Hippocampus as more sensitive to Sox2 loss in comparison to SVZ. Loss of function in mice reproduces that of humans with heterozygous Sox2 mutations including hippocampal abnormalities, epilepsy, motor problems and microphthalmia [7,30,31]. Sox2 is along with Pax6 an important marker stem/progenitor cells in the developing human cerebellum [32].
Sox2 regulatory function is also involved in sight, hearing and smell sensory systems highlighting an additional role as a prosensory factor. Accordingly, mice lacking Sox2 expression present eye defects, hearing loss and abnormal development of the hypothalamopituitary system [33]. It has been also shown that Sox2 is essential for the maintenance of the Retina Neural Precursors (RPCs) selfrenewal capacity as well as for their neurogenic differentiation potential [34]. Similarly, it is well established that Sox2 is implicated in the development of the sensory regions of the inner ear [35- 37] and regulates the emergence of progenitor cells and neuronal differentiation [36]. More specifically, Sox2 mechanism of action involves conjunction with c-Myc to promote proliferation of otic progenitors and direct positive regulation of Atoh1 expression, a hair cell differentiation inducer, in cochlea progenitor cells [38-40]. Thus, Sox2 is involved in the regulation of both neuronal and sensory components of the inner ear, however it also controls the transition from neuronal to hair cell differentiation [41]. The detailed mechanism for this difference is not clear yet, but distinct co-operating factors are good candidates [41]. In terms of olfactory system, Sox2 controls the proliferation of neuronal progenitors in the olfactory epithelium [42]. It is known that in C. elegans Sox2 regulates the specification of terminally differentiated olfactory neurons [43] although such function has not been observed in vertebrates. Finally, Sox2 is also associated with the peripheral nervous system development as it is a marker of Enteric Nervous System progenitor cells and neural crest precursors in hair follicles [44,45]. To conclude, in vivo and in vitro studies reveal the importance of Sox2 in the preservation of NSC properties, including proliferation, self-renewal and neurogenesis (Figure 2).
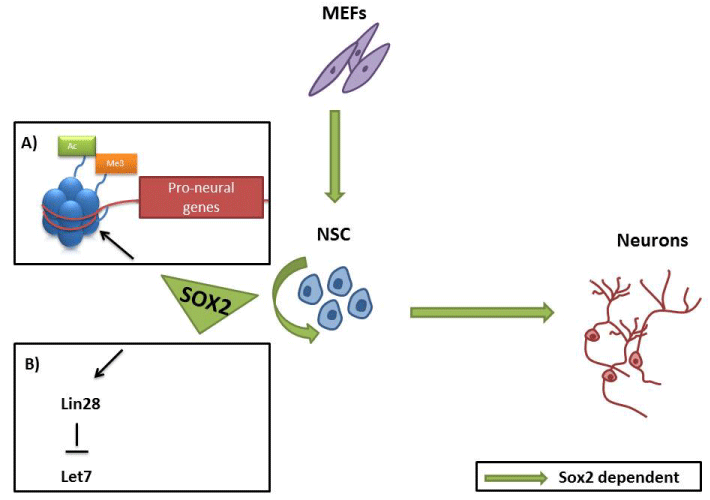
Figure 2: Role of Sox2 in NSC establishment, maintenance and
differentiation. Sox2 promotes self renewal maintenance in NSC through
preservation of pro-neural gene promoters bivalency (A) and up-regulation
of Lin28 expression level (B). Sox2 is sufficient to convert to convert somatic
cells to induced NSC and is required for their efficient neuronal differentiation.
The various outcomes of Sox2 deficiency results mainly from the implicated complex regulatory network. Its mechanism of action is well documented in pluripotent ESC, but is still poorly understood in NSC. Sox2 expression is stringently regulated by different factors including E2fa, E2fb, p21, Pax6, activating protein 2, prospero homeobox protein 1 and Ars2 [46-50]. MiR-145 is also involved in the regulation of NSC properties targeting Sox2 [51]. Additionally, stem cell phenotype is preserved through epigenetic regulation of Sox2 expression. In histone deacetylase 2 (HDAC2) deficient mice impaired deacetylation of Sox2 leads to increased proliferation rate of adult NSC and poor neuronal maturation, unraveling the importance of Sox2 for successful neurogenesis [43,47]. Noticeably, the epigenetic status of Sox2 promoter determines the success of oligodendrocyte precursor to NSC reversion process [52]. On the other hand, limited number of Sox2 responsive genes, including nestin, surviving and sonic hedgehog (Shh), that are essential for NSC maintenance, have been identified [25,53]. Knowing that Sox2 stimulates Shh expression in the hippocampus NPC, a study by Takanaga et al showing that Gli2, a major mediator of Shh signaling pathway, up-regulates Sox2 expression unravels a positive feedback loop mechanism [54]. Furthermore, Sox2 regulates Notch pathway activity through direct up-regulation of Notch1 in retina NPC, whereas only marginal effects on Notch pathway genes were observed in brain NSC upon Sox2 deletion [25,34].
Most of the published data associate Sox2 with self-renewal maintenance, however several studies demonstrated unexpectedly that Sox2 is also important for proper neural differentiation in brain and retina, respectively [34,55]. An additional mechanism of Sox2 action was proposed, involving Lin28/let7 pathway [56]. This mechanism justifies the previous contradictory reports that Sox2 is required for both NSC self-renewal as well as for terminal neuronal differentiation. They showed that Sox2 up-regulates Lin28 expression in NPC through regulation of its promoter acetylation level. Let7 is repressed by Lin28 and becomes expressed upon terminal differentiation of NPC. It was demonstrated that Let7 family members inhibit neural differentiation by repressing the proneural genes Mash1 and Ngn1. In agreement, constitutive expression of Lin28 rescues, whereas ectopic expression of Let7 miRs phenocopies, the loss of Sox2 phenotype in NPC, in terms of proliferation capacity and neuronal differentiation ability [56]. Another report documented the necessity of Sox2 for proper neural differentiation. A novel role for Sox2 as epigenetic modifier in adult hippocampus NSC was uncovered with a combination of in vivo and in vitro deletion experiments [57]. They proved that Sox2 is responsible for preserving the promoter bivalent nature of early expressed pro-neural markers like Ngn2 and NeuroD1. In this system, Sox2 prevents the excessive activity of the repressive polycomb repressive comlex 2 (PRC2) and permits the activation of the genes upon exposure to neurogenic stimulus [57].
Sox2 is generally considered as transcriptional activator in NPC, however recent studies suggest that it can also act as a repressor inhibiting the expression of target genes directly or indirectly. Important for the repressor activity is its interaction with co-factors including co-repressors of the Groucho protein family that are known to affect neurogenesis [58]. Other interactors of Sox2 in NPC include Pax6 in lens and Otx2 in retina [59,60]. Gene ontology analysis of the target genes upon expression array approach gave information about the nature of differentially regulated genes. It was shown that Sox2 regulates the biology of NPC by activating the expression of transcription factors, but silencing the expression of genes associated with mitosis, providing a proliferation rate control mechanism [58].
In summary, Sox2 function in NPC is context- and developmental stage dependent. The various outcome of Sox2 deletion possibly originates from the different expression of Sox2 regulators and interacting partners in different NSC types.
Sox2 in the generation of induced NSC
The combination of increased sensitivity with restricted regeneration ability of neuronal cells predominantly contributes to the development of degenerative disorders, a major spotlight of scientific interest. The main research challenge is the production of new functional neurons in order to replace the lost ones. Neuronal differentiation of pluripotent cells permits generation of neurons for disease modeling studies, yet there are many limitations excluding their use in transplantation therapies. The last years many groups have achieved the generation of post-mitotic neurons directly from somatic cells like fibroblasts, with forced expression of transcription factors and/or microRNAs [61-64]. In recent studies, Sox2 was included in transcription factor combinations used for the production of induced neurons. Zhao and partners showed that Sox2 in conjunction with Ngn2 and Ascl1 can convert human fibroblasts to functional patient specific induced neuronal cells [65]. Additionally, utilization of Sox2 along with other factors has allowed the derivation of induced GABAergic Interneuron’s (iGABA-INs) from mouse and human fibroblasts, that are able to survive upon transplantation into mouse brain [66].
However, both low conversion efficiency and the neuronal low proliferation rate inhibit large-scale production of induced neurons thus restricting their clinical utilization. Furthermore, neurons show low survivability upon transplantation. Hence, recent years studies have focused on the generation of induced NSC (iNSC), which enable expanded production of differentiated cells [67-70]. Their derivation by trans-differentiation of somatic cells is more advantageous in comparison to the commitment of pluripotent ESC/iPSC. Genomic instability of iPSC and possible insufficient silencing of the exogenous c-Myc oncogene expression leads to high risk of tumorigenicity hindering their use in transplantation therapies [71,72]. However no case of teratoma formation has been mentioned when NSC are used. Despite regenerative medicine iNSC are also a valuable tool for disease modeling, drug screening and toxicity tests.
Many studies report induction of NSC from fibroblasts with a combined expression of three or more transcription factors, including Sox2 [67,68,73]. However, most of them are detrimental for clinical applications as some include use of oncogenes, or may produce NSC with limited pluripotency and self-renewal capacity. Additionally, not all of them have achieved reprogramming of human fibroblasts. However, it has been shown that over-expression of Sox2 alone is able to reprogram both human and mouse fibroblasts into iNSC [69]. The generated mouse iNSC can self-renew extensively upon silencing of exogenous Sox2 expression, are able to form functional neurons with synaptic activity in vitro and are multipotent both in vitro and in vivo. Furthermore, they can be transplanted and integrate to the brain without tumor formation. Correspondingly, human iNSC are multipotent with no tumorigenic potential in vivo. Most importantly, a recent study revealed that the expression of Sox2, Brn2 and Foxa2 directed the conversion of fibroblasts into Dopaminergic Precursors (iDP) that could differentiate to dopaminergic neurons upon engraftment into mouse models of Parkinson’s Disease and relieve their motor impairment [74]. Recent studies have expanded the source of starting cell type for this type of experiments. Another work unveiled that Sox2 overexpression in Adipose Tissue-Derived Mesenchymal Stem Cells (ADSCs) leads to iNSC-like cells, which express NSC characteristic markers including Pax6. The ADSCderived NSC-like cells are able to self-renew and differentiate into neuron-like cells, providing another cell source for transplantation therapy [75]. Astrocytes is another cell type amenable to direct conversion into NSC and a new study used inducible expression of Sox2 alone to reprogram astrocytes to NSC [76].
Noticeably, Sox2 is also involved in in vivo reprogramming experiments. Ectopic expression of Sox2 is sufficient to reprogram astrocytes of the brain towards NPC, which can further differentiate to neurons suggesting a new therapeutic approach for degenerative disorders of CNS [77,78]. Su et al utilized Sox2 to reprogram resident astrocytes into doublecortin (DCX)-positive neuroblasts in a model of spinal cord injury [79]. Furthermore, Sox2 is used in in vivo direct conversion experiments as it is able to convert NG2 glia cells into doublecortin (DCX)+ neurons following in vivo injury [80].
To conclude, Sox2 is a well-documented master regulator of NSC, so it is conceivable that its constitutive expression can influence the cell fate of both un- and already differentiated cells towards multipotent NSC, both in vivo and in vitro (Figure 2).
Sox2 in Mesenschymal Stem Cells
During the last decades, Sox2 activity has been also implicated in Mesenchymal Stem Cells (MSC) function, further emphasizing its role in stemness maintenance. MSC are cells of mesodermal origin traditionally found in the bone marrow although alternative sources include umbilical cord, peripheral blood, fallopian tube, fetal liver and lung tissues. Consisting multipotent cells they are able to differentiate towards adipogenic, chondrogenic, and osteogenic pathways. This plastic behavior along with the expression of the pluripotency factors Sox2, Oct4 and Nanog reveals functional similarities with ESC on their maintenance and differentiation capacity.
Sox2 is expressed in a broad range of human MSC and its expression becomes down regulated upon their differentiation. Little is known about regulators of Sox2 expression in MSC with the most representative ones being Oct4 that up-regulates Sox2 expression, resembling ESC and mir-21, which inhibits Sox2 expression in amniotic fluid MSC [81]. Additionally, Yoon and colleagues demonstrated that Sirt1 contributes to MSC self-renewal sustenance through stabilization of Sox2 protein [82]. In MSC, Sox2 functions as a dual regulator of cell cycle progression as well as cell fate determination. More specifically, in human adipose tissue and umbilical cord blood MSC Sox2 positively regulates cell cycle progression by facilitating G1/S transition through transcriptional activation of CyclinD1 and c-Myc [83,84]. In accordance, studies in which Sox2 was overexpressed in the presence of FGF-β or depleted report increased and reduced proliferation capacity, respectively [83,84]. In terms of differentiation decisions, there are contradictory results about the role of Sox2. It has been reported that Sox2 inhibits osteogenic differentiation whereas it is required for efficient adipogenic differentiation of MSC via Dkk1 upregulation and Wnt signaling inhibition [84]. However, another study revealed that Sox2 and Oct4 overexpression increased differentiation ability of human adipose tissue MSC towards both osteogenic and adipogenic differentiation. On the contrary, Schonitzer and colleagues observed that ectopic expression of Sox2 keeps MSC in an undifferentiated state and concomitantly, decreases their osteogenic and adipogenic differentiation potential through negatively regulating Dkk1 expression and activating Wnt signaling pathway. This result was abolished through Sox2 ablation [85]. The aforementioned conflicting studies make clear that more analysis is required in order to elucidate Sox2 mechanism of action on MSC.
Recently, human iPSC have been established with the use of Sox2 from MSC with different origin. Giorgetti and coworkers managed to reprogram Cord Blood (CB) haematopoietic stem cells to iPSC using ectopic expression of Oct4 and Sox2 [86,87]. In addition, the use of Sox2 for iPSC generation from hMSC has been successfully combined with small molecules such as inhibitors for TGFβ receptor- SB 431542, MEK-PD325901, and p53-Pifithrin a.
Sox2 and stem cell therapies
The advent of iPSC technologies has revolutionized human disease modelling, drug testing and ultimately regeneration of tissues for transplantation [82,83]. Sox2 is essential for the establishment of iPSC that have mainly replaced ESC in regenerative medicine [84]. In particular, Sox2 along with Oct4 function as “pioneer” factors due to their ability to access target sites even when they are embedded inside a highly packed chromatin region [85]. Beyond being an essential factor for iPSC derivation, Sox2 is indispensable for proper neurogenesis [86,87].
Neurological diseases are strongly dependent on tissue replacement therapies due to the limited potential for regeneration following aging, stroke or trauma. In accordance with the necessity of Sox2 in the proliferation and maintenance of NSC, it is able to convert, together with additional pro-neural factors, somatic cells to iNs [25,60,61]. Interestingly, Sox2 is also sufficient to switch fibroblasts [64] and ADSC [69] into iNSC thus rendering the procedure simple and safe by the omission of oncogenes. The potential of Sox2 to serve as a NSC regeneration factor was also proven in vivo. Sox2 ectopic expression could regenerate proliferating neuroblasts from residing astrocytes [88]. The feasibility of these induced neuroblasts to differentiate and rescue damaged cortex [71,74] or spinal cord [73] makes Sox2 a very promising target for neuronal degeneration or injury therapies.
Another field where Sox2 may prove useful for human health is sensory tissue regeneration. Even if the importance of Sox2 in RSC self-renewal maintenance and differentiation ability is already well documented, it has not been employed yet for mammalian retina regeneration [31]. In addition, Sox2 is also involved in the development of inner ear sensory regions [33,34]. In contrast to other vertebrates, mammalian cochlea cannot regenerate in mammals in cases of congenital deficiency, damage or aging. Efforts have been made to employ ESC for generation of hair cells [88]. A recent study reports differentiation of hiPSC or ESC towards neurosensory progenitors of Auditory Neurons in vitro that express Sox2 among other neuronal regulators [89]. Sox2 is both an iPSC driving factor and an important regulator of cochlear development therefore it could have future applications in the research aiming to regenerate the auditory system in order to repair hearing loss.
Concerning the peripheral nervous system, Sox2 is a marker of Enteric Nervous System (ENS) progenitor cells and their glial derivatives, thus it is a good candidate molecule for modeling cell replacement therapy for Hirschsprung’s disease (HSCR), which affects ENS [41]. Sox2 is expressed in neural crest precursors and following injury, it is expressed in skin nerve cells that contribute to skin regeneration [42].
Conclusions and Future Directions
Sox2 is a master regulator of stemness in different systems including pluripotent cells and different types of adult stem cells - NSC and MSC - (Figure 3). Its function is indispensable for the establishment of pluripotent ESC in vivo and the generation of iPSC in vitro. This function renders Sox2 a very promising factor for future development of new regenerative medicine tools.
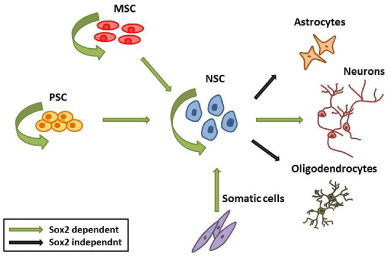
Figure 3: Sox2 promotes self renewal maintenance in ESC, MSC and NSC.
Sox2 positively regulates the establishment of NSC by different cell types
(ESC, MSC, MEFs) and is required for their efficient neuronal differentiation.
Additionally, the importance of Sox2 in the development of central and peripheral nervous system highlights it as an important factor for neuronal tissue regeneration. Sox2 is crucial for the generation of neurons from pluripotent [87,89] and somatic cell types [73,90]. The production of neuronal subtypes that correspond to any given genetic background has recently reformed the efforts to study and fight neurological diseases. In that field Sox2 is involved in the conversion of somatic cells including fibroblasts, astrocytes and pericytes to induced Neurons [60,61,90]. The importance of Sox2 in GABAergic neurons establishment reflects the in vivo situation where hypomorphic or knockout mice have reduced number of GABAergic interneurons [55]. In regenerative medicine Sox2 ability to direct conversion of cells to specific neuronal sub-types should be further examined. The difficulty to expand neurons makes the use of NPC an attractive cell source for their generation. Noteworthy, Sox2 is sufficient to generate NPC from fibroblasts and astrocytes, which permit large scale culture. However, there are still many advantageous cell types that can be tested as starting material including Hematopoietic cells that permit easy isolation and manipulation. Additional functions have been detected for Sox2 in the peripheral nervous system and in sensory tissues specifically in retina [30], inner ear [7,32] and taste bud [91] development and maintenance. However, a concern related to the utilization of Sox2 for human health emerges from the diverse roles it has in cancer and most importantly in cancer stem cells [92].
The molecular mechanisms lying under these distinct functions of Sox2 depend on many factors including expression levels, extracellular signaling, antagonism with tissue specific TFs and partners’ selection in specific cell and developmental stages [93]. Although Sox2 binding loci have been extensively analyzed, many pieces of this complex puzzle are still missing. Future investigations need to elucidate a genome-wide determination of Sox2-interacting partners and a comparative analysis of common target gene loci between Sox2 and synergizing factors.
References
- Kamachi Y, Uchikawa M, Kondoh H. Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet, 2000; 16: 182-187.
- Schepers GE, Teasdale RD, Koopman P. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell. 2002; 3: 167-170.
- Weina K, Utikal J. SOX2 and cancer: current research and its implications in the clinic. Clin Transl Med. 2014; 3: 19.
- Vencken, SF, Sethupathy P, Blackshields G, Spillane C, Elbaruni S, Sheils O, et al. An integrated analysis of the SOX2 microRNA response program in human pluripotent and nullipotent stem cell lines. BMC Genomics, 2014; 15: 711.
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev, 2003; 17: 126-140.
- Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, et al. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development, 2007; 134: 2521-2531.
- Kelberman D, et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest. 2006; 116: 2442-2455.
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005; 122: 947-956.
- Chen X, Vega VB, Ng HH. Transcriptional regulatory networks in embryonic stem cells. Cold Spring Harb Symp Quant Biol. 2008; 73: 203-209.
- Lodato MA, Ng CW, Wamstad JA, Cheng AW, Thai KK, Fraenkel E, et al. SOX2 co-occupies distal enhancer elements with distinct POU factors in ESCs and NPCs to specify cell state. PLoS Genet, 2013; 9: 1003288.
- Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol, 2007. 9: 625-635.
- Aksoy I, Jauch R, Chen J, Dyla M, Divakar U, Bogu GK, et al. Oct4 switches partnering from Sox2 to Sox17 to reinterpret the enhancer code and specify endoderm. EMBO J. 2013; 32: 938-953.
- Kopp JL, Ormsbee BD, Desler M, Rizzino A. Small increases in the level of Sox2 trigger the differentiation of mouse embryonic stem cells. Stem Cells. 2008; 26: 903-911.
- Zhao S, Nichols J, Smith AG, Li M. SoxB transcription factors specify neuroectodermal lineage choice in ES cells. Mol Cell Neurosci. 2004; 27: 332-342.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 2006; 126: 663-676.
- Polo JM., Anderssen E, Walsh RM, Schwarz BA, Nefzger CM, Lim SM, et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012; 151: 1617-1632.
- Buganim Y, Faddah DA, Cheng AW, Itskovich E, Markoulaki S, Ganz K, et al. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell. 2012; 150: 1209-1222.
- Jauch R, Aksoy I, Hutchins AP, Ng CK, Tian XF, Chen J, et al. Conversion of Sox17 into a pluripotency reprogramming factor by reengineering its association with Oct4 on DNA. Stem Cells, 2011; 29: 940-951.
- Pevny L, Placzek M. SOX genes and neural progenitor identity. Curr Opin Neurobiol, 2005. 15: 7-13.
- Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist’s view of neural development. Trends Neurosci, 2005; 28: 583-588.
- Ferri AL, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004; 131: 3805-3819.
- Komitova M, Eriksson PS. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci Lett. 2004; 369: 24-27.
- Episkopou V. SOX2 functions in adult neural stem cells. Trends Neurosci, 2005; 28: 219-21.
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992; 255: 1707-1710.
- Favaro R, Valotta M, Ferri AL, Latorre E, Mariani J, Giachino C, et al. Hippocampal development and neural stem cell maintenance require Sox2- dependent regulation of Shh. Nat Neurosci. 2009; 12: 1248-1256.
- Schmitz M, Temme A, Senner V, Ebner R, Schwind S, Stevanovic S, et al. Identification of SOX2 as a novel glioma-associated antigen and potential target for T cell-based immunotherapy. Br J Cancer. 2007; 96: 1293-1301.
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci, 2003; 6: 1162-1168.
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron, 2003; 39: 749-765.
- Miyagi S, Saito T, Mizutani K, Masuyama N, Gotoh Y, Iwama A, et al. The Sox-2 regulatory regions display their activities in two distinct types of multipotent stem cells. Mol Cell Biol, 2004; 24: 4207-4220.
- Fantes J, Ragge NK, Lynch SA, McGill NI, Collin JR, Howard-Peebles PN, et al. Mutations in SOX2 cause anophthalmia. Nat Genet. 2003; 33: 461-463.
- Sisodiya SM, Ragge NK, Cavalleri GL, Hever A, Lorenz B, Schneider A, et al. Role of SOX2 mutations in human hippocampal malformations and epilepsy. Epilepsia. 2006; 47: 534-542.
- Pibiri V, Ravarino A, Gerosa C, Pintus MC, Fanos V, Faa G. Stem/progenitor cells in the developing human cerebellum: an immunohistochemical study. Eur J Histochem. 2016; 60: 2686.
- Kelberman D, Dattani MT. The role of transcription factors implicated in anterior pituitary development in the aetiology of congenital hypopituitarism. Ann Med. 2006; 38: 560-577.
- Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, et al. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006; 20: 1187-1202.
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, et al. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A. 2008; 105: 18396-18401.
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, et al. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005; 434: 1031-1035.
- Neves J, Vachkov I, Giraldez F. Sox2 regulation of hair cell development: incoherence makes sense. Hear Res. 2013; 297: 20-29.
- Kempfle JS, Turban JL, Edge AS. Sox2 in the differentiation of cochlear progenitor cells. Sci Rep. 2016; 6: 23293.
- Kwan KY, Shen J, Corey DP. C-MYC transcriptionally amplifies SOX2 target genes to regulate self-renewal in multipotent otic progenitor cells. Stem Cell Reports, 2015; 4: 47-60.
- Puligilla C, Kelley MW. Dual role for Sox2 in specification of sensory competence and regulation of Atoh1 function. Dev Neurobiol. 2016.
- Raft S, Groves AK. Segregating neural and mechanosensory fates in the developing ear: patterning, signaling, and transcriptional control. Cell Tissue Res. 2015; 359: 315-332.
- Packard AI, Lin B, Schwob JE. Sox2 and Pax6 Play Counteracting Roles in Regulating Neurogenesis within the Murine Olfactory Epithelium. PLoS One, 2016; 11: 0155167.
- Alqadah A, Hsieh YW, Vidal B, Chang C, Hobert O, Chuang CF, et al. Postmitotic diversification of olfactory neuron types is mediated by differential activities of the HMG-box transcription factor SOX-2. EMBO J. 2015; 34: 2574-2589.
- Heanue TA, Pachnis V. Prospective identification and isolation of enteric nervous system progenitors using Sox2. Stem Cells. 2011; 29: 128-140.
- Johnston AP, Naska S, Jones K, Jinno H, Kaplan DR, Miller FD. Sox2- mediated regulation of adult neural crest precursors and skin repair. Stem Cell Reports. 2013; 1: 38-45.
- Andreu-Agullo C, Maurin T, Thompson CB, Lai EC. Ars2 maintains neural stem-cell identity through direct transcriptional activation of Sox2. Nature. 2011; 481: 195-198.
- Jawerka, M., Colak D, Dimou L, Spiller C, Lagger S, Montgomery RL, et al. The specific role of histone deacetylase 2 in adult neurogenesis. Neuron Glia Biol. 2010; 6: 93-107.
- Julian LM, Vandenbosch R, Pakenham CA, Andrusiak MG, Nguyen AP, McClellan KA, et al. Opposing regulation of Sox2 by cell-cycle effectors E2f3a and E2f3b in neural stem cells. Cell Stem Cell. 2013; 12: 440-452.
- Lengler J, Bittner T, Münster D, Gawad Ael-D, Graw J. Agonistic and antagonistic action of AP2, Msx2, Pax6, Prox1 AND Six3 in the regulation of Sox2 expression. Ophthalmic Res, 2005; 37: 301-309.
- Marques-Torrejon MA, Porlan E, Banito A, Gómez-Ibarlucea E, Lopez- Contreras AJ, Fernández-Capetillo O, et al. Cyclin-dependent kinase inhibitor p21 controls adult neural stem cell expansion by regulating Sox2 gene expression. Cell Stem Cell. 2013; 12: 88-100.
- Xu, N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009; 137: 647-658.
- Lyssiotis CA, Walker J, Wu C, Kondo T, Schultz PG, Wu X. Inhibition of histone deacetylase activity induces developmental plasticity in oligodendrocyte precursor cells. Proc Natl Acad Sci U S A. 2007; 104: 14982-14987.
- Tanaka S, Kamachi Y, Tanouchi A, Hamada H, Jing N, Kondoh H. Interplay of SOX and POU factors in regulation of the Nestin gene in neural primordial cells. Mol Cell Biol. 2004; 24: 8834-8846.
- Takanaga H, Tsuchida-Straeten N, Nishide K, Watanabe A, Aburatani H, Kondo T. Gli2 is a novel regulator of sox2 expression in telencephalic neuroepithelial cells. Stem Cells. 2009; 27: 165-174.
- Cavallaro M, Mariani J, Lancini C, Latorre E, Caccia R, Gullo F, et al. Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development. 2008; 135: 541-557.
- Cavallaro M, Mariani J, Lancini C, Latorre E, Caccia R, Gullo F, et al. Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development. 2008; 135: 541-557.
- Cimadamore F, Amador-Arjona A, Chen C, Huang CT, Terskikh AV. SOX2- LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc Natl Acad Sci U S A. 2013; 110: 3017-3026.
- Amador-Arjona A, Cimadamore F, Huang CT, Wright R, Lewis S, Gage FH, et al. SOX2 primes the epigenetic landscape in neural precursors enabling proper gene activation during hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2015; 112: 1936-1945.
- Liu YR, Laghari ZA, Novoa CA, Hughes J, Webster JR, Goodwin PE, et al. Sox2 acts as a transcriptional repressor in neural stem cells. BMC Neurosci. 2014; 15: 95.
- Danno H, Michiue T, Hitachi K, Yukita A, Ishiura S, Asashima M. Molecular links among the causative genes for ocular malformation: Otx2 and Sox2 coregulate Rax expression. Proc Natl Acad Sci U S A. 2008; 105: 5408-5413.
- Kamachi Y, Uchikawa M, Tanouchi A, Sekido R, Kondoh H. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev. 2001; 15: 1272-1286.
- Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011; 476: 224-227.
- Ladewig J, Mertens J, Kesavan J, Doerr J, Poppe D, Glaue F, et al, Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Methods. 2012. 9: 575-578.
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, et al. Induction of human neuronal cells by defined transcription factors. Nature, 2011; 476: 220-223.
- Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A. 2011; 108: 10343-10348.
- Zhao P, Zhu T2, Lu X3, Zhu J2, Li L. Neurogenin 2 enhances the generation of patient-specific induced neuronal cells. Brain Res. 2015; 1615: 51-60.
- Colasante G, Lignani G, Rubio A, Medrihan L, Yekhlef L, Sessa A, et al. Rapid Conversion of Fibroblasts into Functional Forebrain GABAergic Interneurons by Direct Genetic Reprogramming. Cell Stem Cell. 2015; 17: 719-734.
- Kim J, Lignani G, Rubio A, Medrihan L, Yekhlef L, Sessa A, et al. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A. 2011; 108: 7838-7843.
- Lujan E, Chandaa S, Ahleniusa H, Südhof TC, Werniga M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A. 2012; 109: 2527-2532.
- Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012; 11: 100-109.
- Sheng, C, Zheng Q, Wu J, Xu Z, Wang L, Li W, et al. Direct reprogramming of Sertoli cells into multipotent neural stem cells by defined factors. Cell Res. 2012; 22: 208-218.
- Liang Y, Zhang H, Feng QS, Cai MB, Deng W, Qin D, et al. The propensity for tumorigenesis in human induced pluripotent stem cells is related with genomic instability. Chin J Cancer, 2013; 32: 205-212.
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007; 448: 313-317.
- Han, DW, Tapia N, Hermann A, Hemmer K, Höing S, Araúzo-Bravo MJ, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012; 10: 465-472.
- Tian C, Li Y, Huang Y, Wang Y, Chen D, Liu J, et al. Selective Generation of Dopaminergic Precursors from Mouse Fibroblasts by Direct Lineage Conversion. Sci Rep. 2015; 5: 12622.
- Qin Y, Zhou C, Wang N, Yang H, Gao WQ. Conversion of Adipose Tissue- Derived Mesenchymal Stem Cells to Neural Stem Cell-Like Cells by a Single Transcription Factor, Sox2. Cell Reprogram. 2015; 17: 221-226.
- Poulou M, Mandalos NP , Karnavas T , Saridaki M , McKay RD , Remboutsika E. A “Hit and Run” Approach to Inducible Direct Reprogramming of Astrocytes to Neural Stem Cells. Front Physiol, 2016; 7: 127.
- Niu W, Zang T, Smith DK, Vue TY, Zou Y, Bachoo R, et al. SOX2 reprograms resident astrocytes into neural progenitors in the adult brain. Stem Cell Reports. 2015; 4: 780-794.
- Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, et al. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol, 2013; 15: 1164-1175.
- Su Z, Niu W, Liu ML, Zou Y, Zhang CL. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun. 2014; 5: 3338.
- Heinrich C, Bergami M, Gascón S, Lepier A, Viganò F, Dimou L, et al. Sox2- mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Reports. 2014; 3: 1000-1014.
- Trohatou O, Zagoura D, Bitsika V, Pappa KI, Antsaklis A, Anagnou NP, et al. Sox2 suppression by miR-21 governs human mesenchymal stem cell properties. Stem Cells Transl Med. 2014; 3: 54-68.
- Yoon DS, Choi Y, Jang Y, Lee M, Choi WJ, Kim SH, et al. SIRT1 directly regulates SOX2 to maintain self-renewal and multipotency in bone marrowderived mesenchymal stem cells. Stem Cells, 2014; 32: 3219-3231.
- Han SM, Han SH, Coh YR, Jang G, Ra JC, Kang SK, et al. Enhanced proliferation and differentiation of Oct4- and Sox2-overexpressing human adipose tissue mesenchymal stem cells. Exp Mol Med. 2014; 46: 101.
- Park SB, Seo KW, So AY, Seo MS, Yu KR, Kang SK, et al. SOX2 has a crucial role in the lineage determination and proliferation of mesenchymal stem cells through Dickkopf-1 and c-MYC. Cell Death Differ. 2012; 19: 534- 545.
- Schonitzer V, Wirtz R, Ulrich V, Berger T, Karl A, Mutschler W, et al. Sox2 is a potent inhibitor of osteogenic and adipogenic differentiation in human mesenchymal stem cells. Cell Reprogram. 2014; 16: 355-365.
- Giorgetti A, Montserrat N, Rodriguez-Piza I, Azqueta C, Veiga A, Izpisúa Belmonte JC. Generation of induced pluripotent stem cells from human cord blood cells with only two factors: Oct4 and Sox2. Nat Protoc. 2010; 5: 811- 820.
- Huang Y, Tan S. Direct lineage conversion of astrocytes to induced neural stem cells or neurons. Neurosci Bull. 2015; 31: 357-367.
- Cimadamore F, Fishwick K, Giusto E, Gnedeva K, Cattarossi G, Miller A, et al. Human ESC-derived neural crest model reveals a key role for SOX2 in sensory neurogenesis. Cell Stem Cell. 2011; 8: 538-551.
- Tian C, Li Y, Huang Y, Wang Y, Chen D, Liu J, et al. Selective Generation of Dopaminergic Precursors from Mouse Fibroblasts by Direct Lineage Conversion. Sci Rep. 2015; 5: 12622.
- Barlow LA. Progress and renewal in gustation: new insights into taste bud development. Development. 2015; 142: 3620-3629.
- Hadjimichael C, Chanoumidou K, Papadopoulou N, Arampatzi P, Papamatheakis J, Kretsovali A. Common stemness regulators of embryonic and cancer stem cells. World J Stem Cells. 2015; 7: 1150-1184.
- Sarkar A, Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013; 12: 15-30.