Abstract
A replacement series study was conducted to evaluate the competition between rice and Caesulia axillaris Roxburgh or Echinochloa crus-galli (L.) Beauv at two nutrient levels to investigate that how differences in nutrient availability may change the competitive relationship between rice and weeds. Plants were established in mixture proportions of 4:0, 3:1, 2:2, 1:3 and 0:4 (weed: rice) plants pot-1. Both weeds were more competitive than rice under high nutrients. C. axillaris exhibited higher Relative Yield (RY) as well Aggressivity (Agr) than rice, whereas E. crus-galli showed comparable RY but greater competitive ability (Agr) than rice. Further, nutrient stress had different effects on both weeds; although, nutrient stress decreased the competition intensity of C. axilaris against rice, it did not change its position in hierarchy (C. axilaris dominated rice). Whereas E. crus-galli was outcompeted by rice under low nutrient. Thus, our study showed that weeds are better at high nutrient than are the crop. However, the effect of nutrient on weed competitiveness is not straightforward rather it depends on growth traits and nutrient use efficiency of species.
Keywords: Aggressivity; Competitive ability; Replacement series; Rice; Weed
Introduction
Weeds are one of the most important biological constraints in rice production [1,2]. According to study by, rice research directorate, India [3], grain yield losses due to weeds in these rice fields ranged from 15% to 90%. Studies indicate that among grasses Echinochloa crus-galli and broad-leaved weeds Caesulia axillaris are the most serious weeds of rice under rice–wheat system of the Indian subcontinent [4-6]. E. crus-galli, a vigorous C4 annual species, is one of the world’s most serious grassy weed in rice [7,8]. This species is highly competitive, and its morpho-physiological similarities with rice make control measures difficult [9]. Its infestation in the rice field results in the reduced rice tillering, which results in loss of yield [10] because it strongly competes with the rice for soil N by removing up to 80% of it [10,11]. The aggressiveness of E. crus-galli is probably due to its efficient C4 photosynthetic pathway, high nitrogen and water use efficiency [12]. In contrast, Caesulia axillaris (pink node flower) is C3 broad-leaved weed of family Asteraceae. It is an annual weed, with extended emergence period, relatively fast growth and high seed production; in these studies, it reduced the rice yield by 33% [13,14].
Weed management decisions for a particular species can be derived from a detailed knowledge of its biology and competitive ability [15]. Therefore, understanding the relationship between plant traits and competitive ability has emerged as a major research area [16,17]. Such an understanding would provide a better insight into the processes shaping the vigour of weeds. Generally, plant biomass, height and leaf area has been used to provide information on the size and aggressiveness of the plant, which may determine its competitive ability [18]. In recent years, physiological basis for competitive ability offered a powerful means to predict the consequences of competitive interaction. The physiological traits such as Specific Leaf Area (SLA) and photosynthetic rate (Aarea), are thought to be linked with resource capture and use efficiency [19]. Differential photosynthetic responses to changes in environmental factors such as nutrient, light can affect species aggressivity. However, little is known about the photosynthetic activity of E. cruss-gali and C. axillaris growing along with rice.
The competitive performance of weeds and crop often depends on environmental conditions [20]. The resources and the fluctuations in their availability can also play a significant role in the competitive ability of species [21,22]. It has been recognized that among the major resources (light, nutrient and water) competition in rice field between weeds and crop are greatest for nutrients [6,14]. Because in tropics, the majority of rice crop grown in lowland areas by transplanting method (rice field is flooded throughout the crop growth period until the grain matuaration), and as summer crop [5,6]. Therefore, in these areas, water and light were never a limiting factor. It has been found that added nutrients increased the competitive ability of weeds more than their crop [23,24]. Conversely, some studies showed that growth of some weed species is decreased while that of the crop is favored under high soil nutrient levels [25,26]. C. axillaris and E. cruss-galli are documented as fast growing and nitrophilic species [7]. Singh et al. [27] reported that fertilization increased the biomass of C. axillaris and E. crus-galli more than that of rice; however, the biomass of same weeds was decreased as compared to the crop at a low dose of N fertilizer. Therefore, it is important to investigate that how altered nutrient availability may change the competitive relationship between rice and weeds and which plant traits are the most important to determine competitive ability of weeds and crop. In order to do so for rice agroecosystem, we designed a replacement series study to elucidate how C. axillaris and E. crus-galli respond in terms of biomass production and physiological performance, when in competition with rice under different nutrient ability. The objectives of the present study was: (i) to study the responses of E. crus-galli and C. axillaris (including physiological performance, biomass accumulation and partitioning) and rice to one another, and (ii) to elucidate how nutrient stress alters the competitive performance of the weeds (iii) identification of plant trait associated with competitive ability of plant. We hypothesized that weeds would be more competitive than crop at high nutrient but that the crop would outcompete the weeds under low nutrient conditions.
Materials and Methods
The competitive interference of Caesulia axillaris Roxburgh and Echinochloa crus-galli (L.) Beauv against rice (BPT var.) was studied in a replacement series in an ambient light-temperature condition (natural weather condition) in the green house at the Botanical Garden of Banaras Hindu University Varanasi, (25°15'N latitudes and 80°59’E longitudes) from July to October 2011.
Treatments and experimental design
Treatments comprised growing the test crop (rice) and weeds (C. axillaris and E. crus-galli) in pure stand and, in mixed cultures; with two strength of nutrient solution viz. 50% Hoagland’s (HN treatment) and 25% Hoagland’s nutrient solution (LN treatment). Hoagland’s solutions were prepared according to Arnon and Hoagland [28]. The full strength (l/l) solution has a nitrogen concentration of 3mm Ca(NO3)2.4H2O and 2mm KNO3. Five planting ratios of the two species used in the study were 4:0, 3:1, 2:2, 1:3 and 4:0. Treatments were replicated five times in a randomized complete block design. For calculations, we report only three replicates for each treatment because some pots were damaged providing a lower number of replicates per treatment. Thus to maintain uniformity, data measured from three replicates. In some mixture proportion where no damage was recorded, we had taken result from all five replicates, but it was not significantly different from the data measured at three replicates. The final sizes of the plants in HN treatments were consistent with the range of sizes observed in the field, suggesting that the nutrient levels were within the range that these species experienced in the field. To ensure that planting density would be enough to result in interference; pilot studies were conducted before deciding to set total plant density in monocultures at four plants pot-1. Species were grown from seeds, collected from a nearby agricultural field. Seeds were germinated on moist filter paper in petridishes at 25°C before the experiment started germination rates were above 90%. To avoid the replacement series experiment problem of initial size bias [29], all seedlings were used in the experiment of similar size. Plants were grown in plastic pot (20-cm-diam by 18-cm-deep). Each pot was filled to a depth of 15 cm with fine river sand. Sufficient water per day and 100 ml of Hoagland nutrient solution at the interval of three days were provided to assure normal plant growth. Pots were flushed once a month with distilled water to prevent salt accumulation. Supplementary watering without nutrients was applied during the evening. Each pot is covered with perlite (about 2.0 cm) to reduce the compaction caused by watering and evaporation from soil surface. Pots were re-randomized weekly to avoid the creation of microclimates and species in mixtures were mixed uniformly and distributed equidistantly in the sand.
Plants were harvested at 70 days after transplantation when physiological maturity achieved in plants. Three replicates were collected for each species in each treatment. Shoots and roots were separated and placed in separate paper bags and transported to the laboratory in ice bags. Harvested plant parts were then dried in an oven at 80°C for 48 h. From the biomass data, the aggressivity of the species towards each other and relative yield and Relative Yield Total (RYT) of each species combination were computed. Leaf Area (LA) was determined using a leaf area meter (SYSTRONICS, Leaf area meter-211). Specific Leaf Area (SLA) (cm2 g-1) was calculated as area per unit mass. Leaf Area Ratio (LAR) was calculated as the ratio of leaf area to plant weight. LA, SLA and LAR are widely used variable in comparative plant ecology, because they are associated with many important attributes of plant growth and survival. A superior SLA may increase the capacity of the plant to assimilate CO2 because more leaves are produced for a given mass of carbon invested in photosynthetic tissues [30,31]; and therefore, provides a higher rate of return on the resources invested when compared to species with a lower SLA. For herbs and grasses, variation in LAR is the key determinant of interspecific variation in RGR [32]. Thus, the more plant invests in leaf area, the higher the total carbon gain and the faster growth will be achieved.
Photosynthetic rate (Aarea), the plants was measured by LI-6400 gas exchange system (LI-COR, Lincoln, Nebraska, USA) on the upper-most, fully expanded and apparently healthy leaves from each individual on sunny days in natural light condition between 0800 and 1100 hours local time. Flow rate was maintained at 500-μmol s-1. Air temperature was 32 ± 0.15°C and CO2 concentration was 385±5 μmol CO2 mol-1. Photosynthetic Active Radiation (PAR) was 1221±36.20 μmol mol-1 at the time of experiment. Higher rates of photosynthesis can lead to increased growth rates, biomass accumulation and overall production [33]. Additionally, high carbon gain and growth may confer high competitive ability to species so that they easily out compete the slow growing species by facilitating colonization or resource acquisition [30].
Competition indices
Relative Yield (RY) and Relative Yield Total (RYT):
RY = Yab/Yaa
RYba = Yba/Ybb
In this study, RY and RYT were calculated by whole plant dry weight data. Yab is the total biomass production of species ‘a’ in a mixture with species ‘b’ and Yaa is the biomass production of species ‘a’ in monoculture. Yab/Yaa is relative yield of species ‘a’ in mixture with species ‘b’ and vice versa; Yba is the total biomass production for species ‘b’ in mixture with species ‘a’, and Ybb is the biomass production of species ‘b’ in monoculture.
RYT is a measure of resource complementarity. RYT value of 1.0 indicates that the two species have equal demands for the same limiting resources of the environment. A RYT value greater than 1.0 means that, although the species may compete for the same resources, they also make demands on different resources. A RYT value less than 1.0 indicates mutual antagonism [34,35].
In replacement diagrams, actual RY of each species was plotted against the appropriate planting proportion. Expected RY for a species occurs when plants of this species grow equally well in mixture and monoculture. Comparisons of actual RY of each species with their expected RY (diagonal dashed line in replacement diagrams) indicate: (1) competition if the actual RY curve of one species is concave and that of the second convex, (2) niche differentiation if actual RY curves of both species are convex, or (3) mutual antagonism if actual RY curves of both species are concave. If actual RY curves are linear (i.e., do not differ from expected), the ability of one species to interfere with the other is equivalent.
Aggressivity (Agr): It is an index for the measure of the intensity of plant competition. It is used by McGilchrist & Trenbath [36] for the first time. Gain or loss of biomass due to interspecific competition was determined by calculating Aggressivity (Agr) for each species. A dominant species will have a higher aggressivity index than a dominated species [37].
Aab = (Yab/Yaa×Zab) – (Yba/Ybb×Zba)
Where Yab, Yba, Yaa, and Ybb are as defined in previous equation. Zab and Zba are sown proportions of crop “a” and “b” in the mixture. If Aab=0, both species are equally competitive, and if Aab is positive then species ‘a’ is the dominant species, while a negative value for Aab means that ‘a’ is the dominated species and vice versa.
Statistical analysis
An analysis of variance (Procedures in SPSS 17.0) was used to partition the main effects of species, mixture ratios, nutrient level. RY and RYT from each mixed culture were compared to the value of 1.00 using t-tests (P=0.05). The data were log-transformed before analysis to normalize statistical distributions and meet with the assumptions of the ANOVA.
Results
Biomass production
ANOVA indicated that species, nutrient treatment and the mixture proportion had significant effects on plant biomass. A summary of the analysis of variance of the effects of species, nutrient level, mixture ratio is presented in (Table 1).
Species (F3,60)
Treatment (F1,60)
Mixture (F3,60)
Leaf Area (LA)
1432***
379***
164***
Specific Leaf Area (SLA)
1548***
87.4***
2.7
Leaf Area Ratio (LAR)
441***
48.4***
1.10
Photosynthetic Rate (Aarea)
20.7***
250.9***
22.2***
Biomass
410.7***
236.5***
193.6***
Root Weight Ratio (RWR)
47.2***
130.0***
0.9
Leaf Weight Ratio (LWR)
21.6***
44.7***
0.9
Relative Yield (RY)
15.02***
19.1***
314.8***
Relative Yield Total (RYT)
14.8***
22.6***
1.3
Aggressivity (Agr)
96.4***
21.8***
126.8***
***Significant at P< 0.01 level, ** significant at P< 0.05 level.
Table 1: Summary (F and P values) of analysis of variance for the effects of species, mixture ratios and nutrient treatments on ecophysiological and biomass partitioning parameters.
In monoculture, C. axillaris had significantly greater biomass than rice at the end of the experiment, and biomass production per pot of C. axillaris was greater than that of rice by 125 % under High Nutrient (HN) treatment, and 105 % under Low Nutrient (LN) treatment (Figure 1). However, E. crus-galli had almost similar biomass to rice. Nutrient stress had great negative effect on biomass production of all studied species. Compared with the HN treatment, LN treatment decreased the DW of C. axillaris by 44%, E. crus-galli by 45% and rice by 32%, respectively (Figure 1). Lines with constant slopes would present equal competition between species across all ratios, resulting in an intersection point of the two curves at the 2:2 ratio. As shown in (Figure 1) the plot of C. axillaris and rice interaction did not intersect at the 2:2 ratio, indicating frequent occurrence of interspecific competition where growth of rice was restrained in the mixtures. However, the plot of E. crus-galli and rice showed almost constant slope and intersect at the 2:2 ratio, reveals that relative gain in biomass of one species in a mixture was equal to loss in a biomass of other species. Consequently, the sum of biomass of E. crus-galli and rice at each proportion was similar to that of each species grown in monoculture. It implies that both species are equivalent in competitiveness (i.e. rice could be substituted for E. crus-galli or E. crus-galli for rice on an equal basis, with a similar effect on biomass production).
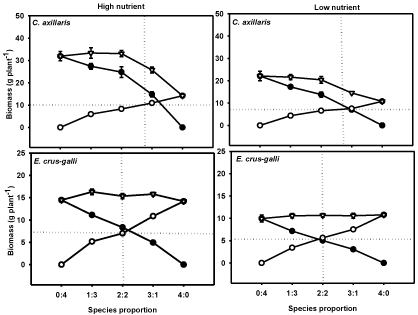
Figure 1: Biomass production of the C. axillaris (●), E. crus-galli (●), rice
(O) and total biomass per pot (∇) at different mixture ratios under High
Nutrient (HN), and Low Nutrient (LN) treatments. Vertical lines are standard
errors. Two straight lines in each frame indicate the theoretically expected
responses for two equally competitive species, which intersect at the point of
equivalency, Harper [51].
Relative Yield (RY) and Relative Yield Total (RYT)
Relative Yield (RY) differed significantly among treatment, mixture proportion and species; however, RYT varied only in treatment and species (Table 1). The replacement diagram illustrated in Figure 2 is interpreted based on the shape of the curves derived from the dry weight of each species. Actual RY of C. axillaris was significantly higher than expected in each proportion under HN whereas actual RY of rice did not differ from expected with the exception of 1:3 ratio. The plot of RY data of C. axillaris and rice intersect at the left of the 2:2 mixture (Figure 2), indicating that C. axillaris was a better competitor of resources than rice. Conversely, under LN the lines intersect almost at the point of equivalency (2:2 mixture ratios) of the expected yield, demonstrating that the weed and rice have a relatively similar interspecific effect on biomass production of one another.
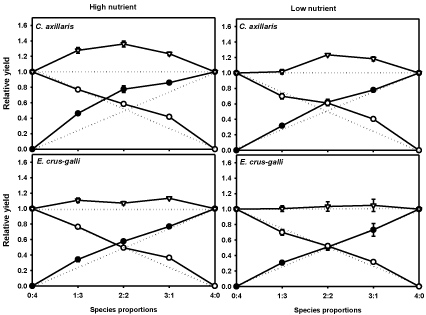
Figure 2: Replacement series diagrams illustrating mean ± SE relative yield
of C. axillaris (●), E. crus-galli (●), rice (O) and relative yield total (∇) as a
function of species proportions. The diagonal dashed lines are the expected
relative yields when plants of a species grow equally well in mixture and
in monoculture of C. axillaris, E. crus-galli and rice under different nutrient
treatments.
Actual RY of E. crus-galli and rice, when grown together, were not significantly differ from expected values in each mixture proportion under LN treatment. However, it was little higher from expected values in E. crus-galli under HN treatment. The plot of RY data of E. crus-galli and rice intersected almost at the point of equivalency in both nutrient treatments, indicating that both species were equally competitive for resources.
Mixtures were over yielding i.e. RYT was higher than 1.0 under HN regardless of weed species. Whereas, under low nutrient, RYT was near unity for E. crus-galli and rice mixture while significantly higher than 1 for C. axillaris and rice mixture except in the 1:3 mixture ratio (weed:rice).
Competitive ability
Aggressivity (Agr) an index to calculate competitive ability, differed significantly between species and nutrient treatments (Table 1). C. axillaris and E. crus-galli had a positive and rice a negative aggressivity with the exception at 1:3 (weed: rice) planting ratio, suggesting that both weeds were dominant and rice the subordinate species under high nutrient treatment (Table 2). Aggressivity of C. axillaris was greater than that of E. crus-galli under both nutrient treatments. Aggressivity values of C. axillaris and E. crus-galli declined as its density decreased in the mixture, and were negative at the weed: crop planting ratio of 1:3. Low nutrient significantly reduced the aggressivity values of both weeds and even negative value observed for E. crus-galli at 2:2 ratio (Table 2).
CA × Rice
ECg × Rice
Weed:rice ratio
HN
LN
HN
LN
3 : 1
2.15 ± 0.09
1.93 ± 0.02
1.94 ± 0.08
1.68 ± 0.12
2 : 2
0.55 ± 0.03
0.03 ± 0.01
0.17 ± 0.06
-0.03 ±0.01
1 : 3
-0.92 ± 0.07
-1.15 ± 0.14
-1.95 ± 0.02
-1.79 ± 0.12
Table 2: Aggressivity (mean ± SE) of C. axillaris (CA), E. crus-galli (ECg), when grown with rice at different mixture ratios under different nutrient treatments.
Growth attributes
We observed significant species, mixture and nutrient treatment effects on LA; however, LAR and SLA showed only species and treatment effects (Table1). C. axillaris showed greater LA, SLA and LAR than rice in mixture; however, E. crus-galli exhibited less LA, SLA and LAR as compared to rice in mixture under both nutrient treatments (Table 3).
Traits
SLA
LAR
Treatments
HN
LN
HN
LN
CAmonoculture
324 ± 1.2
318 ± 1.8
87 ± 17.1
70 ± 4.7
CA3:Rice1
325 ± 0.8
317 ± 2.5
80 ± 3.9
73 ± 4.8
CA2:Rice2
330 ± 7.5
322 ± 1.5
77 ± 11.6
68 ± 3.3
CA1:Rice3
318 ± 1.8
319 ± 1.6
75 ± 8.9
67 ± 7.1
Ricemonoculture
170 ± 1.5
166 ± 0.2
35 ± 5.8
28 ± 3.9
Rice3: CA1
170 ± 0.8
168 ± 1.5
39 ± 1.6
29 ± 2.8
Rice2: CA2
171 ± 1.8
165 ± 1.0
35 ± 2.6
27 ± 3.5
Rice1: CA3
170 ± 0.7
164 ± 1.3
41 ± 7.5
21 ± 1.3
ECgmonoculture
144 ± 5.0
126 ± 1.7
23 ± 1.4
18 ± 3.3
ECg3 : Rice1
133 ± 1.4
127 ± 1.1
23 ± 1.5
19.9 ± 4
ECg2 : Rice2
129 ± 1.5
128 ± 1.0
21 ± 3.0
18 ± 2.3
ECg1 :Rice3
128 ± 0.2
131 ± 1.1
22 ± 1.3
16 ± 3.7
Rice3 : ECg1
170 ± 0.9
168 ± 1.5
34 ± 2.4
29 ± 5.3
Rice2 :ECg2
172 ± 1.1
165 ± 0.6
38 ± 2.8
28 ± 3.7
Rice1 :ECg3
171± 2.4
163 ± 1.0
37 ± 3.0
32 ± 4.9
Table 3: Specific Leaf Area (SLA; cm2 g-1) and Leaf Area Ratio (LAR; cm2 g-1) of C. axillaris (CA), E. crus-galli (ECg) and rice at different mixture ratios under High Nutrient (HN) and Low Nutrient (LN) treatment.
Leaf area of the both weeds declined linearly as the number of rice plant increased in the pot. In C. axillaris, the decrease in LA in mixture with rice than monoculture was higher in low nutrient (43%) as compared to high nutrient (36%), while E. crus-galli showed almost similar decrease in both nutrient treatments. The plot of LA of rice and C. axillaris intersects at the right of the point of equivalence i.e. at 3:1 planting ratio (rice: C. axillaris) in both nutrient treatments (Figure 3), again showing the dominance of C. axillaris in the mixture. However, the plot of E. crus-galli and rice intersect close to the 2:2 ratio in both nutrient treatments.
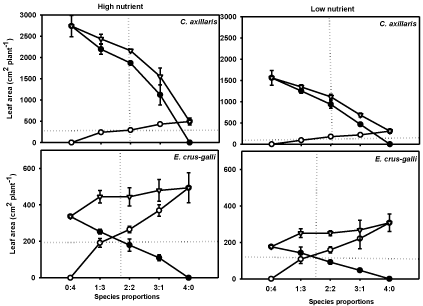
Figure 3: Leaf area of C. axillaris (●), E. crus-galli (●), rice (O) and total leaf
area per pot (∇) at different mixture ratios under High Nutrient (HN), and Low
Nutrient (LN) treatments. Vertical lines are standard errors. Two straight lines
in each frame indicate the theoretically expected responses for two equally
competitive species, which intersect at the point of equivalency, Harper [51].
Physiological attributes
Species, treatment and mixture significantly influenced photosynthetic rate (Aarea ) (Table 1). Both weeds showed higher Aarea as compared to rice in mixture under HN. Under LN, Aarea of C. axillaris reduced but were never lower than rice; however, E. crus-galli exhibited lower Aarea than that of rice (Figure 4). Both weeds showed larger reduction in Aarea under LN, while rice showed greater decrease under HN treatment. The step-wise multiple regressions revealed that among the growth attributes and biomass accumulation, Leaf Area (LA) had the largest influence on competitive ability (Agr) of plants. It was observed that the variation in LA accounted for 54.5% variation in Agr. The final model was Agr = -0.22LA + 0.74 (R2 = 0.54 P = 0.04).
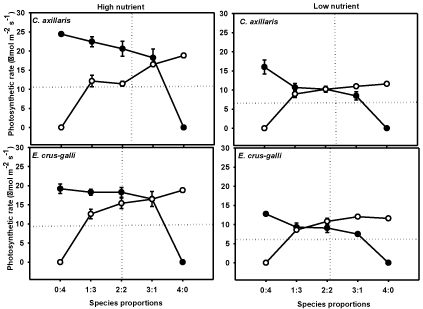
Figure 4: Photosynthetic rate of C. axillaris (●), E. crus-galli (●) and rice (O)
at different mixture ratios under High Nutrient (HN), and Low Nutrient (LN)
treatments. Vertical lines are standard errors. Two straight lines in each frame
indicate the theoretically expected responses for two equally competitive
species, which intersect at the point of equivalency, Harper [50].
Discussion
The species with the greater competitive ability is usually termed as dominant species or superior competitor, and has a greater ability to acquire resources and to occupy the superior ecological niche [38]. Positive Agr value indicates increased ability for competition; therefore, C. axilaris and E. crus-galli were more competitive and rice was less competitive when grown in equal proportion. However, data on the relative yield reveals that C. axilaris was a superior competitor when grown in mixture with rice while, there was equal competition between E. crus-galli and rice. Therefore, more than one approach for determining competitiveness is required. Interspecific competition was greater at HN than LN treatment, which suggests that the intensity of competition enhanced with increasing resource availability [39]. Further, the result indicated greater competitive ability of C. axilaris than E. crus-galli with rice. Rapid development due to superior growth traits of C. axilaris at early growth stages was likely to responsible for its competitive advantage. The nutrient stress did change the competition intensity between C. axilaris and rice, but did not change the position in hierarchy i.e. C. axilaris holds a competitive advantage over rice. Conversely, the value of aggressivity for E. cruss-galli was negative, implies that it was dominated by rice in pot. One feasible explanation for the reduced competitiveness of E. cruss-galli in low nutrient may be that E. cruss-galli competitiveness is highly dependent on nitrogen as also found in the study of Holm et al. [7].
The RYT gives an accurate assessment of the greater biological efficiency in competitive situation [40]. The RYT value of mixtures in HN treatment were greater than 1.0, regardless of the planting ratio and weed type, indicating that while the two species competed for resources, but complementary facilitation dominated over the competitive interference. Moreover, RYT values higher than 1.0 and lower than 2.0 indicate that resource complementarity and competition interference between the crop and weeds occurred at the same time [41]. Other studies on crop and weed mixtures have also attributed values of RYT > than 1 [42-44]. The mixture of E. crusgalli and rice showed RYT close to one (> 1.2) in HN and equal to 1 in LN (Figure 2), which showed that both the species required the same limiting resources for successful growth. It could be possibly due to the similarity in their growth habit, rooting architecture, shoot and leaf morphology as both species in mixture belong to same life form (grasses). Usually, species with similar growth habits could make similar demands on the limited resource for growth, but the differences in their efficiency in utilization of these resources makes them a better competitor [45]. Further, values of RYT always greater than 1 in C. axilaris-rice mixture, implies that the crop exploited the resources somewhat differently than did C. axilaris, probably, due to different rooting depths between the two species as both species belongs to distinct life form.
Understanding of the trait-specific growth parameters aids the explanation of potential mechanisms by which resource exploitation can provide competitive ability [46]. Several traits are known to be affect biomass accumulation and thereby competitive ability viz. RGR, Aarea, SLA and LA [46,47]. There was considerable variation among the three species for each growth traits; C. axilaris showed significantly higher values of SLA, LA and Aarea, among all. This result is consistent with the results of competitive indice, where C. axilaris exhibited highest value of Agr. In this instance, the high competitive ability (Agr) of C. axilaris observed in this study can be explained in terms of its prolific rooting system [27], which enables to capture more of soil water and nutrients. In addition, high LA and SLA enables it to produce more assimilates by providing greater surface area for photosynthesis which translated into high RGR. Moreover, although E. crus-galli had relatively comparable biomass and relative yield in the mixture with rice under HN, it exhibited greater competitive ability than rice. Therefore, it can be speculated that comparatively higher photosynthetic rate than rice could be responsible for its increased competitive potential. Conversely, despite of relatively similar biomass to rice, these traits were lower in LN treatment, which results in negative Agr than rice. This result supports the view of Weigelt et al. [48], who reported that competitive strength of a species could be determined through species-specific traits and biomass allocation strategies.
Stepwise multiple regressions were performed to determine which growth parameter influenced the aggressivity most. In this study, stepwise regression picked up LA as the most important trait, which accounted for the greatest amount of variability in Agr, emphasizing the role of leaf area in determining the competitive potential of rice and weeds across the gradients of nutrient availability. Rapid leaf area development in plants is beneficial for light competition [1]; in addition, high leaf area contributes to competitiveness by increasing growth rate, which resulted in rapid biomass accumulation of the plant [1,49,50]. However, leaf area could explain only 54.5% variability in Agr, indicating that other traits, not studied by us are also important in modulating the competitive ability of weeds and rice.
Conclusion
This study showed that nutrient availability had different effects on the different species. C. axilaris was the superior competitor than rice at both higher and lower nutrient levels. Although nutrient stress decreased the competition intensity of C. axilaris against rice, it did not change its position in the hierarchy. Unlike C. axilaris, E. crusgalli performed poorly in nutrient stress conditions (as shown by the competitive indices) and was outcompeted by rice. Thus, our study is consistent with the hypothesis that weeds are better at high nutrient than are the crop. However, contrary to our expectations, the low nutrient had little effect on C. axilaris competitiveness; only E. crusgalli showed a decrease in competitiveness. Thus, it appeared that the effect of nutrient on weed competitiveness is not straightforward rather it depends on growth traits and nutrient use efficiency of species.
Acknowledgement
VS thank the Council of Scientific and Industrial Research, India for financial support as a Senior Research Fellow (Extended).
References
- Ni H, Moody K, Robles RP, Paller EC, Lales JS. Oryza sativa plant traits conferring competitive ability against weeds. Weed Sci. 2000; 48: 200-204.
- Chauhan BS, Johnson DE. Responses of rice flatsedge (Cyperus iria) and barnyardgrass (Echinochloa crus-galli) to rice interference. Weed Sci. 2010; 58: 204-208.
- Rice knowledge management portal. Directorate of rice research Hyderabad. 2011.
- Singh A, Sharma GP, Raghubanshi AS. Dynamics of the functional groups in the weed flora of dryland and irrigated agroecosystems in the Gangetic plains of India. Weed Biol. Manag. 2008; 8: 250-259.
- Singh S, Ladha JK, Gupta RK, Bhushana L, Rao AN. Weed management in aerobic rice systems under varying establishment methods. Crop Prot. 2008; 27: 660-671.
- Subhas C, Jitendra P. Effect of rice (Oryza sativa) culture, nitrogen and weed control on nitrogen competition between scented rice and weeds. Indian J Agron. 2001; 46: 68-74.
- Holm LG, Plucknett DL, Pancho JV, Herberger JP. The world's worst weeds. Distribution and biology. Krieger Publishing Company, Malabar, Florida. 1991; 609.
- Rao AN, Johnson DE, Sivaprasad B, Ladha JK, Mortimer AM. Weed management in direct-seeded rice. Adv Agron. 2007; 93: 153-255.
- Galon L, Agostinetto D. Comparison of empirical models for predicting yield loss of irrigated rice (Oryza sativa) mixed with Echinochloa spp. Crop Prot. 2009; 28: 825-830.
- Holm LG, Plucknett DL, Pancho JV, Herberger JP. The world's worst weeds distribution and biology, University Press of Hawaii, Honolulu, Hawaii. 1977; 609.
- Chauhan BS, Johnson DE. Ecological studies on Echinochloa crus-galli and the implications for weed management in direct-seeded rice. Crop Prot. 2011; 30: 1385-1391.
- Bouhache M, Bayer DE. Photosynthetic response of flooded rice (Oryza sativa) and three Echinochloa species to changes in environmental factors. Weed Sci. 1993; 41: 611-614.
- Bhowmik PC. In: Proceeding International Symposium Integrated Weed Management for Sustainable Agriculture, Hisar. 1993; 57-66.
- Lakhwinder LS, Kolar JS, Brar LS. Critical period of competition between Caesulia axillaris Roxb. and transplanted rice. Indian J Weed Sci. 1995; 27: 154-157.
- Booth BD, Swanton. Assembly Theory Applied to Weed Communities. Weed Sci. 2002; 50: 2-13.
- Storkey J. Modelling seedling growth rates of 18 temperate arable weed species as a function of the Environment & plant traits. Ann Bot. 2004; 93: 681-689.
- Navas ML. Trait-based approaches to unravelling the assembly of weed communities & their impact on agro-ecosystem functioning. Weed Res. 2012; 52: 479-488.
- Radosevich S, Holt J, Ghersa C. Weed Ecology. New York. J Wiley. 1997; 589.
- Osunkoya O, Bayliss D, Dane Panetta FD, Smith GV. Leaf trait co-ordination in relation to construction cost, carbon gain and resource-use efficiency in exotic invasive and native woody vine species. Ann Bot. 2010; 106: 371-380.
- Patterson DT. Effects of environmental stress on weed/crop interactions. Weed Sci. 1995; 43: 483-490.
- Davis MA, Grime JP, Thompson K. Fluctuating resources in plant communities: a general theory of invasibility. J Ecol. 2000; 88: 528-534.
- Davis M A, Pelsor M. Experimental support for a resource based mechanistic model of invasibility. Ecol Lett. 2001; 4: 421-428.
- Blackshaw RE, Brandt, RN, Janzen HH, Entz T, Grant CA, Derksen DA. Differential response of weed species to added nitrogen. Weed Sci. 2003; 51: 532-539.
- Blackshaw RE, Molnar LJ, Janzen HH. Nitrogen fertilizer timing and application method affect weed growth and competition with spring wheat. Weed Sci. 2004; 52: 614-622.
- Abouziena H F, El-Karman, M F, Singh M, Sharma S. Effect of nitrogen rates and weed control treatments on maize yield and associated weeds in sandy soils. Weed Technol. 2007; 21: 1049-1053.
- Cathcart RJ, Swanton CJ. Nitrogen management will influence threshold values of green foxtail (Setaria viridis) in corn. Weed Sci. 2004; 51: 975-986.
- Singh V, Singh H, Raghubanshi AS. Effect of N application on emergence and growth of weeds associated with rice. Trop Ecol. 2017; 58.
- Arnon DI, Hoagland DR. Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci. 1940; 50: 463-483.
- Connolly J, Goma HC, Rahim K. The information content of indicators in intercropping research. Agric Ecosyst Environ. 2001; 87: 191-207.
- Lambers H, Poorter H. Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Adv. Ecol Res. 1992; 23: 188-261.
- Reich PB, Walters MB, Ellsworth DS. From tropics to tundra: global convergence in plant functioning. P Natl Acad Sci. 1997; 94; 13730-13734.
- Atkin OK, Botman B, Lambers H. The causes of inherently slow growth in alpine plants: an analysis based on the underlying carbon economies of alpine and lowland Poa species. Funct Ecol. 1996; 10: 698-707.
- Allred BW, Fuhlendorf SD, Monaco TA, Will RE. Morphological and physiological traits in the success of the invasive plant Lespedeza cuneata. Biol Invasion 2010; 12, 739-749.
- Bi HQ, Turvey ND. Inter-specific competition between seedlings of Pinus radiata, Eucalyptus regnans and Acacia melanoxylon. Aust J Bot. 1994; 42: 61-70.
- Keddy PA, Twolan-Strutt L, Wisheu I. Competitive effect and response rankings in 20 wetland plants: are they consistent across three environments? J Ecol. 1994; 82: 635-643.
- Mcgilchrist CA, Trenbath BR. A revised analysis of plant competition experiments. Biometricsl. 1971; 27: 659-671.
- Snyder KM, Baskin JM Baskin CC. Comparative ecology of the narrow endemic Echinacea tenneis and two geographically widespread congeners: relative competitive ability and growth characteristics. Int J Plant Sci. 1994; 155: 57-65.
- Zhang G, Yang Z, Dong S. Interspecific competitiveness affects the total biomass yield in an alfalfa and corn intercropping system. Field Crop Res. 2011; 124: 66-73.
- Hartvigsen G. Competition between co-dominant plants of the Serengeti plains depends on competitor identity, water and urine. Plant Ecol. 2000; 148: 31-41.
- Xu B, Xu W, Huang J, Shan L, Li F. Biomass production and relative competitiveness of a C3 legume and a C4 grass co-dominant in the semiarid Loess Plateau of China. Plant Soil. 2011; 347: 25-39.
- Mariotti M, Masoni A, Ercoli L, Arduini I. Above- and below-ground competition between barley, wheat, lupin and vetch in a cereal and legume intercropping system. Grass Forage Sci. 2009; 64: 401-412.
- Estorninos LE, Gealy DR, Talbert RE. Growth response of rice (Oryza sativa) and red rice (O. sativa) in a replacement Series Study. Weed Technol. 2002; 16: 401-406.
- Gealy DR, Estorninos LE, Gbur EE, Chavez RSC. Interference interactions of two rice cultivars and their F3 cross with barnyard grass (Echinochloa crus-galli) in a replacement series study. Weed Sci. 2005; 53: 323-330.
- Singh V, Singh H, Raghubanshi AS. Competitive interactions of wheat with Phalaris minor or Rumex dentatus: A replacement series study. Int J Pest Manag. 2013. 59: 245-258.
- Tuor FA, Froud-Williams RJ. Interaction between purple nutsedge, maize and soybean. Int J Pest Manage. 2002; 48: 65-71.
- Roush ML, Radosevich SR. Relationships between growth and competitiveness of four annual weeds. J Appl Ecol. 1985; 22: 895-905.
- Pywell RF, Bullock JM, Roy DB, Warman L, Walker KJ, Rothery P. Plant traits as predictors of performance in ecological restoration. J Appl Ecol. 2003; 40: 65-77.
- Weigelt A, Steinlein T, Beyschlag W. Does plant competition intensity rather depend on biomass or on species identity? Basic Appl Ecol. 2002; 3: 85-94.
- Reich PB, Walters MB, Ellsworth DS. Leaf life span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol Monogr. 1992; 62: 365-392.
- Reich PB. Reconciling apparent discrepancies among studies relating life span, structure and function of leaves in contrasting plant life forms and climates: ‘The blind men and the elephant retold’. Funct Ecol. 1993; 7: 721-725.
- Harper JL. Plant Population Biology. Academic Press, San Diego, USA. 1977.
