Abstract
Jute is most commonly used natural fiber as reinforcement in green composites. It is one of the low-cost natural fiber and is presently the bast fiber with the maximum production volume. Jute has potential to cope Copper (Cu) stress due to its huge biomass. Therefore, present experiment was conducted to investigate which varieties of jute can tolerate under Cu stress. Moreover, Cu sensitive varieties also determined from the present experiment. For this purpose, a petri dish experiment was conducted under different levels of Cu i.e. 0 (control), 2, 5, 10, 20 and 50 μmol/L using Da An Qing Pi variety then different varieties of jute undergo 50μmol/L Cu and growth parameters and seed germination was assayed. Results revealed that 50μmol/L of Cu affected seed germination and plant biomass and increased the activity of Superoxidase Dismutase (SOD), Peroxidase (POD), affected most by the Cu stress indicating the oxidative stress which is manifested by high Malondialdehyde (MDA) and proline contents also. Shang Huo Ma and Gu Ba Chang Jia are sensitive under Cu stress while Hong Tie Gu Xuan and C-3 tolerate under the Cu stress without significant reduction in plant height, plant fresh weight, root length, shoot length, plant dry weight and shoot dry weight. Thus based on the results, it can be concluded that Hong Tie Gu Xuan and C-3 are Cu resistant varieties while Shang Huo Ma and Gu Ba Chang Jia are Cu sensitive varieties.
Keywords: Jute; Copper stress; Morphology; Antioxidants enzymes; Proline; Copper resistant varieties; Copper sensitive varieties
Introduction
Heavy metals are significant environmental pollutants, and their toxicity is a problem of increasing significance for ecological, evolutionary, nutritional and environmental reasons. Heavy metals include Lead (Pb), Cadmium (Cd), Nickel (Ni), Cobalt (Co), Iron (Fe), Zinc (Zn), Chromium (Cr), Iron (Fe), Arsenic (As), Silver (Ag), Copper (Cu) and the platinum group elements. Anthropogenic activities such as industrial effluents, mining, and sewage sludge as well as fertilizers and pesticides application are the major sources of heavy metal accumulation in soils [1]. Copper (Cu) is among the abundant heavy metals in agricultural soils. Cu-based pesticides, in particular bactericides, fungicides, and herbicides, are widely used in agricultural practice throughout the world. However, concentration of Cu within cellular components need to be maintained at low level because toxic level of Cu can induce alterations in photosynthesis, respiration, enzyme activity, DNA, and membrane integrity leading towards inhibited growth and endangered survival of plants [2]. Cu is also an essential component of various proteins like plastocyanin of photosynthetic system and cytochrome oxidase of respiratory electron transport chain [3]. Copper (Cu) is essential for plants, listed in period 4 and group IB of the periodic table with atomic number 29, atomic weight 63.5, and density 8.96 g cm-3, having boiling point 2595oC and melting point 1083oC [4]. Plants suffered from Cu toxicity appear stunted, usually bluish in color, and eventually turned yellow or brown. The presence of heavy metals, e.g. Cu in the soil at higher rates, significantly reduce the plant productivity and crop yields [5]. Toxic concentration of Cu in soil can cause nutrient imbalance by binding with organic matter, clay minerals, and hydrated oxides of Iron (Fe), Aluminum (Al), and Manganese (Mn), which affects the plant productivity [6]. Excess of Cu resulted in inhibited plant growth as well as impairment of cellular processes, e.g. photosynthetic electron transport [7]. Copper toxicity in Rhodes grass (Chloris gayana) damaged the plant roots, with symptoms ranging from disruption of root cuticle and reduced root hair proliferation, to severe deformation of root structure [8]. Although jute can cope Cu toxicity and able to survive on Cu contaminated soil due to its huge biomass.
Copper in excess causes generation of ROS such as superoxide radical (O-), H2O2, singlet oxygen (1O2), and hydroxyl radicals (OH [9]. Antioxidant enzymes such as Superoxidase Dismutase (SOD) and Peroxidase (POD) are involved in the scavenging of ROS. The SOD catalyzes the disputation of superoxide to H2O2 and molecular oxygen, whereas POD decomposes H2O2 by oxidation of cosubstrates, such as phenolic compounds and or antioxidants [10]. Plants exposed to excess copper have been shown to accumulate proline in their tissues [11]. Accumulation of proline is an adaptive response of plants against stresses. Proline is believed to be regulatory or signal molecule activating some physiological and molecular responses [12]. The hydroxyl radicals react with biological molecules, including unsaturated fatty acid from membrane to form lipid radicals along with a cytotoxic product Malondialdehyde (MDA), which is an indicator of free radical production and consequent tissue damage [13].
Jute is most commonly used natural fiber as reinforcement in green composites. Jute is a type of bast fibers from Tiliaceae family and having scientific name is Corchorus capsularis because it is extracted from plants of corchorus. It is one of the low-cost natural fiber and is presently the bast fiber with the maximum production volume [14]. Jute is intuitive to the Mediterranean but now a days Bangladesh, India, China, Nepal, Thailand, Indonesia, and Brazil provide the finest type for the growth of jute [15]. Jute is also known also golden fiber due to its versatile nature. Jute can grow 2-3.5 m in height and are very brittle, with a low extension to break because of the high lignin content (up to 12-16%). Jute fibers have a less resistance to moisture, acid and UV light. The jute industry has special importance in the economy of India and continues to be a major traditional earner of foreign exchange. However, it is facing tough competition from the synthetic fibers. Conversely, their fine texture as well as their resistance to heat and fire are providing a widespread range of applications in industries such as textile, construction, and automotive Jute fiber composites have become more popular from the last few decades because it is of light weight and can be process easily. The most communal natural fibers used as bast fibers, such as hemp, jute, flax, kenaf, and sisal etc. The two main species of jute plants are: Corchorus cupsuluris (white jute) and Corchorus olitorius (tossa). The fiber, which forms only a small portion of the jute plant (5-6 % of the green weight), is located between the outer bark and the central pith, or stick. The fiber is extracted from the jute plant by steeping in water (retting). Due to retting, the cementing materials undergo bacterial decomposition, which loosens the fiber from the surrounding cortical tissue, thereby enabling the fiber to be separated from the plant. Different varieties of jute shown in Table 1.
Number
Varieties
Origin
1
Min-49 Hong
China
2
Min 208
China
3
Long Xi Hong Pi
Unknown
4
Hong Tie Gu Xuan
Unknown
5
C-3
Bangladesh
6
Diao Wu Huang Ma
Unknown
7
YongTai Huang Ma
Unknown
8
Tu Huo Ma
Unknown
9
Guang Ba Ai
Unknown
10
Yin Du Mo Lv Zi
Unknown
11
Da An Qing Pi
Unknown
12
Gu Ba Chang Jia
Unknown
13
Yong Tai Ge Ling Ma
Unknown
14
Min-26
China
15
Shang Huo Ma
Unknown
16
Hun Xuan19
Unknown
17
Yun Xiao Hong Pi
Unknown
18
Yong An Hong Pi
Unknown
19
Ping He Dan Zhu
Unknown
20
HeZi No.8
Unknown
Table 1: Presenting different varieties of jute.
The uniqueness of jute due to its high biomass production and tolerance towards Cu can be valuable traits for phytoremediation capability; however, sufficient information is not available regarding Cu tolerance and antioxidative defense system. Therefore, the present study was planned to investigate the effects of different concentrations of Cu on growth, lipid peroxidation, and antioxidant enzymatic activities in jute varieties. Keeping in view the importance of jute present experiment was planned to explore the effect of different concentrations of copper on the germination and morphology. Moreover, different varieties of jute were analyzed to find the Cu resistant and Cu sensitive varieties. .According to best of our knowledge, this study is among the few studies which focus on the metal tolerance and antioxidants capacity of different varieties of jute. Findings from the present study will add to our understanding the mechanism of Cu tolerance of different varieties of jute.
Materials and Methods
Plant growth conditions
Jute seeds were used for the petri dish experiments (Table 1). Seeds collected from different research institutes of Hubei province and were surface sterilized with 0.1% HgCl2 for the prevention of surface fungal/bacterial contamination (Young, 1926). Seeds of Da An Qing Pi were undergo under different levels of Cu i.e. 0 (control), 2, 5, 10, 20 and 50 μmol/L that were prepared in pure distilled water in laboratory by using copper sulphate (CuSO4.5H2O) 99% pure. Experiment was done in germinating machine with 30oC and 80% humidity with 12 h artificial light in January 2018. Forty seeds were placed on double filter paper in each petri dish and 5ml of solution was used as prepared above. The experiment was designed complete random with three replications of each treatment. The fresh solutions were applied every alternate day for the prevention of contaminants and also for the maintenance of concentration. The nutrient solution was provided once in a week, replacing the copper treatment for 24 h. The petri dishes were monitored daily for fungal and other type of infections. The plants were harvested after 14 days of seed sowing and sampling was took for enzymatic study of jute. After that different varieties of jute were treated by using highest treatment of the copper (50μmol/L). The growth parameters like seed germination, shoot length, root length, total length, shoot fresh weight, total weight, shoot dry weight and total dry weight were measured after 14 days of seed sowing. All chemicals used were of excellent quality and taken from Sinopharm Chemical Reagent Co., Ltd.
Seed germination and growth parameters
Seed germination were noted after five days of seed sowing. The seeds were considered as germinated when the shoots were grown more than 2mm [16]. Total length was measured from root to shoot tip of the plants. Then shoot length and root length also measured by separated the shoot with the root. Also, the total weight was measured from weight balance (Shimadzu AY-220) by measuring the total fresh weight of the plant. Shoot fresh weight and root fresh weight was also measured. Then plants were over dried at 105oC for 1 h and 65oC for 72 h. Then total dry weight and shoot dry weight was measured with the help of weighting balance (Shimadzu AY-220).
Antioxidants enzymatic activities
In order to check enzymes activities, fresh leaves (0.5g) were homogenized in liquid nitrogen and 5mL of 50mmol sodium phosphate buffer (pH 7.0) including 0.5mmol EDTA and 0.15mol NaCl. The homogenate was centrifuged at 12000×g for 10 min at 4oC and the supernatant was used for measurement of SOD and POD activities.
The SOD activity was assayed in 3mL reaction mixture containing 50mM sodium phosphate buffer (pH 7), 56mM Nitroblue Tetrazolium (NBT), 1.17mM riboflavin, 10mM methionine, and 100μL enzyme extract. Finally, reading was taken by using spectrophotometer (xMarkTM micro plate absorbance spectrophotometer, BIO-RAD, USA). The method was followed by Chen and Pan [17] and expressed in mg g-1 Fresh Weight (FW).
POD activity in leaves was estimated using the method of Thomas et al. [18] and was assayed using guaiacol as the substrate. The reaction mixture (3mL) contained 0.05mL of enzyme extract, 2.75ml of 50mM phosphate buffer (pH 7.0), 0.1 mL of 1% H2O2, and 0.1 mL of 4% guaiacol solution. The increase in the absorbance at 470nm due to guaiacol oxidation was recorded for 2min. One unit of enzyme activity was defined as the amount of the enzyme causing a change in absorbance of 0.01 per minute. The specific POD activity was expressed as U g-1 FW min-1.
Lipid peroxidation and proline content
For the analysis of chlorophyll contents, 0.1g of fresh leaf sample was extracted with 8 mL 95% acetone for 24 h at 4oC in darkness. The absorbance was measured by a spectrophotometer (Shimadzu UV-2550, Kyoto, Japan) at 646.6, 663.6, and 450 nm. Chlorophyll contents were calculated by the standard method of Porra et al., (1989) and expressed in mg g-1 Fresh Weight (FW).
The degree of lipid peroxidation was evaluated as Malondialdehyde (MDA) contents. 0.1g of frozen leaves was ground at 4oC in a mortar with 25mL of 50mM phosphate buffer solution (pH 7.8) containing 1% Polyethene Pyrrole (PVP). The homogenate was centrifuged at 10,000×g at 4oC for 15 min. The mixtures were heated at 100oC for 15-30 min and then quickly cooled in an ice bath. The absorbance of the supernatant was recorded by using spectrophotometer (xMarkTM microplate absorbance spectrophotometer, BIO-RAD, USA) at the wavelengths of 532, 600, and 450 nm. Lipid peroxidation was expressed as l molg-1 using the following formula: 6.45 (A532 - A600) - 0.56 A450. The method was followed by Porra et al. [19] and expressed in mg g-1 Fresh Weight (FW).
Proline contents were measured by using (0.1g) homogenate in 3% of aqueous sulphosalicylic acid and distilled water. The proline content was assessed by the technique described by Bates et al. [20] and expressed in mg g-1 Fresh Weight (FW). The soluble protein content was determined according to Bradford (1976) and expressed in mg g-1 Fresh Weight (FW).
Statistical analysis
All the results were given as arithmetic means with standard deviations except otherwise defined. Data were tested with one-way ANOVA, followed by LSD tests using Statistix 8.1. The significance level was set at P‹0.05 or P‹0.01. Graphical presentation was carried out using Graph pad prism 6.
Results and Discussion
Effect of Cu stress on seed germination
The present study investigated the seed germination and morphological changes of different varieties of jute under Cu stress. Cu has been used as heavy metal for different kinds of morphological and physiological assays due to its both types of effects i.e. essential and toxic effect on plants [21]. Therefore, a preliminary experiment was conducted on germination of jute seedling under Cu stress. Germination assays is an important parameter to determine the effect of metal stress on the plant seedlings [22]. Table 2 and Table 3 showed the germination of Da An Qing Pi and different varieties of jute. Seed germination was noted on fifth day after the seed sowing. Different treatments of Cu showed different results. The germination rate of the jute seedlings significantly decreased with the increase in the Cu concentrations, ranging from 0 to 50μmol/L while the germination rate for 0μmol/L is maximum after 5th day from the beginning of the experiment i.e. 95% and the germination rate of highest treatment (50μmol/L) was minimum i.e. 50%. While the germination of different varieties of jute show the different germination. All varieties of jute show the germination percentage ranging from 75-100%. The highest germination percentage was observed in Hong Tie Gu Xuan and C-3 while the least showed by Gu Ba Chang Jia and Shang Huo Ma. The germination of jute decreases as the Cu stress increases, which show the Cu partitioning during the germinating stage [23]. Similar results were found by Ahsan et al. 2007 [16]. When they studied effect of Cu stress under rice germination. On the other hand, Hong Tie Gu Xuan and C-3 show the maximum seed germination under stress condition showing proper adaptation of the seedling [24]. Similar results were showed by Nasri et al. 2015 [25] who demonstrated that environmental stress might be reduced seed germination in Dodonaea viscosa L. However, Gu Ba Chang Jia and Shang Huo Ma showed the minimum germination under the high Cu stress showed that enable to survive on Cu stress environment.
Treatment (μmol/L)
Germination Percentage
0
100
2
92.5
5
85
10
67.5
30
60
50
50
Table 2: Effect of Cu stress on germination of Da An Qing Pi seedling. Relative radiance of plastic filter used: 0, 2, 5, 10, 30 and 50μmol/L.
Varieties
Germination Percentage %
Min-49 Hong
82.5
Min 208
92.5
Long Xi Hong Pi
87.5
Hong Tie Gu Xuan
100
C-3
100
Diao Wu Huang Ma
87.5
Yong Tai Huang Ma
85
Tu Huo Ma
92.5
Guang Ba Ai
92.5
Yin Du Mo Lv Zi
100
Da An Qing Pi
95
Gu Ba Chang Jia
75
Yong Tai Ge Ling Ma
92.5
Min-26
80
Shang Huo Ma
77.5
Hun Xuan 19
77.5
Yun Xiao Hong Pi
75
Yong An Hong Pi
80
Ping He Dan Zhu
90
He Zi No.8
85
Table 3: Effect of Cu stress on germination of different varieties of jute. Relative radiance of plastic filter used different varieties of jute.
Effect of Cu stress on antioxidants, proline and Malondialdehyde (MDA) on Da An Qing Pi variety
The effect of Cu stress on antioxidants i.e. SOD and POD are shown in the Figure 1. The results showed that as the Cu concentration increases the activity of SOD and POD also increases. The maximum increase in the SOD activity was 29% at 50μmol/L Cu when compared to 0μmol/L Cu. The maximum SOD activity was observed at 50μmol/L Cu (47U g-1 FW) while the minimum SOD activity was observed at 0μmol/L Cu (14U g-1 FW). In the same way, the maximum POD activity was increased by 46% at 50μmol/L Cu when compared to 0μmol/L Cu. The effect of different Cu levels on MDA and proline was showed in Figure 2. The results showed that the MDA contents increases as the Cu levels increases showed oxidative damage in the leaves. The maximum increased of MDA contents in the leaves by 31% at 50μmol/L Cu when compared to 0μmol/L Cu. In the same way maximum increase of proline contents by 94% at 50μmol/L Cu when compared to 0μmol/L Cu. The contents of proline ranges from 48.6μmol g-1 FW at 0μmol/L Cu to 51.4μmol g-1 FW at 50μmol/L Cu.
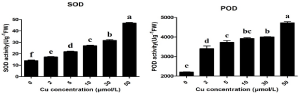
Figure 1: Influence of different Cu treatments on SOD (a) and POD (b) in
Da An Qing Pi jute variety. Letters indicate statistical differences (p=0.05)
according to an HSD test. n = 3.
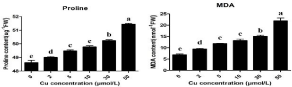
Figure 1: Influence of different Cu treatments on Proline (a) and MDA (b)
in Da An Qing Pi jute variety. Letters indicate statistical differences (p=0.05)
according to an HSD test. n = 3.
The antioxidants play an important role when plants undergo any environmental stress. Also the oxidative damage is also linked with the environmental stresses [26]. ROS usually generated by normal cellular activities like photorespiration but its concentration in the plants increases too much when plants undergo any environmental stress. The antioxidants enzymes such as SOD and POD are involved in the scavenging of Reactive Oxygen Species (ROS) [27]. Under high environmental stress the antioxidants enzymes such as SOD and POD can prevent the formation of ROS species which causes damage to the plants [28]. Many previous reports suggested that the activity of antioxidants enzymes SOD and POD and contents of proline of MDA and proline increased when the plants undergo Cu stress. In the present study Cu stress also induced the increase in the enzymatic activity of SOD and POD which noticed maximum at 50μmol/L Cu (Figure 1). The role of POD also very important in many developmental processes which involve in the scavenging of H2O2 molecules in the leaves [29]. In the present study POD activity was maximum at 50μmol/L Cu which showed similar findings from [30] when he used biochar under Cu stress by using Boehmeria nivea L. Determination of of Malondialdehyde (MDA) amount is used to measure the extent of lipid peroxidation [31]. In the current study, MDA contents were minimum at 0μmol/L Cu and maximum at 50μmol/L Cu hence showed the similar results founded by [32] when they used moso bamboo under hydroponic Cu stress and MDA contents were increased by 84% at 100μmol/L Cu when compared to 0μmol/L Cu. Under environmental stresses plant accumulate a large amount of proline in their leaves which tolerate them from toxic effect of stress [13]. Studied Boehmeria nivea L. using different levels of Cu in pot experiment and found that the proline contents increases continuously as the Cu levels increases. Hence, proline was maximum at 400mg/kg of Cu when compared to 0mg/kg. All these results depicted that at 50μmol/L Cu jute undergo high stress and enhances the enzymatic activities of SOD and POD and oxidative compounds i.e. proline and MDA to reduce Cu toxicity.
Effect of Cu stress on plant growth and biomass
Results regarding the plant lengths and biomass are showed in the table 4 and table 5. All growth parameters were taken after 14 days of seed sowing. All the data taken of seedling height was greatly affected by different levels of Cu stress while different varieties of jute also affected by high Cu stress. Results showed that increase in the Cu stress continuously decreases the plant height, fresh and dry biomass. At the 14th day of the experiment plant height was found (5.2cm) which show the normal growth while Hong Tie Gu Xuan and C-3 reached a height of 3.7cm and 3.8cm due to high stress of Cu. Total height of plant was decreased by 48% at 50μmol/L Cu when compared to 0μmol/L Cu. After 14 days of seed sowing the seedling height of Hong Tie Gu Xuan and C-3 are statistically similar with each other showed excellent growth and development while the seedling height of Shang Huo Ma and Gu Ba Chang Jia are also statistically similar with each other showed poor growth and development under Cu stress. Fresh and dry weight of seedling also reduced as the increase in the Cu stress. Different levels of Cu stress had significant effect on seedling fresh biomass. Seedling fresh weight decreased by 63% at 50μmol/L Cu when compared to 0 μmol/L Cu while dry biomass was reduced by 37% at 50μmol/L Cu when compared to 0μmol/L Cu. Fresh biomass was ranged from (1.1-0.7 g) for different treatments of Cu stress while for different varieties it ranges from (1.7-0.6 g). All these results showed that jute is strongly affected by Cu stress. While on the other hand different varieties of jute showed different results under Cu stress. Hence seedling dry biomass also influenced by different levels of Cu and different varieties of jute. The lowest dry biomass (0.03g) was found at 50μmol/L Cu while the maximum (0.08g) dry biomass was found at 0μmol/L Cu showed Cu stress induced jute dry biomass. On the other hand maximum dry biomass was found in C-3 and Hong Tie Gu Xuan varieties i.e. 0.09g while minimum dry biomass was found in Shang Huo Ma and Gu Ba Chang Jia i.e. 0.01g. The highest seedling length, fresh and dry biomass was showed by Hong Tie Gu Xuan and C-3 in all aspects while Shang Huo Ma and Gu Ba Chang Jia showed the poor growth under the Cu stress.
Cu (μmol/L)
Seedling height (cm)
Seeding fresh weight (g)
Seedling dry weight (g)
0
5.6±0.2 a
1.1±0.05 a
0.08±0.005 a
2
4.6±0.2 b
1±0.05 ab
0.07±0.005 ab
5
4.2±0.1 c
1±0.05 b
0.06±0.001 b
10
3.6±0.2 d
0.8±0.05 c
0.05±0.005 c
30
3.3±0.2 d
0.8±0.1 c
0.04±0 c
50
2.7±0.2 e
0.7±0.05 c
0.03±0.005 d
Table 1: Effect of different levels of Cu stress on Seedling height, Seeding fresh weight and Seedling dry weight in Da An Qing Pi. Values in the table is just one harvests ± SD (n =3). Different letters within a column indicate significant difference between the treatments (P‹0.05 or P‹0.01). Relative radiance of plastic filter used: 0, 2, 5, 10, 30 and 50μmol/L.
Varieties
Seedling height (cm)
Seeding fresh weight (g)
Seedling dry weight (g)
Min-49 Hong
3.1±0.1 b
0.9±0.05 fgh
0.06±0.005 b
Min 208
2.3±0.1 g
1.2±0.05 cde
0.05±0.005 bc
Long Xi Hong Pi
1.6±0.15 j
1.2±0.05 cde
0.06±0.01 b
Hong Tie Gu Xuan
3.7±0.15 a
1.7±0.05 b
0.09±0.005 a
C-3
3.8±0.1 a
1.6±0.05 a
0.09±0.01 a
Diao Wu Huang Ma
3±0.1 c
0.7±0.1 hij
0.04±0.005 cd
Yong Tai Huang Ma
2.8±0.1 d
1±0.05 def
0.04±0.005 de
Tu Huo Ma
2.5±0.1 f
0.9±0.05 fgh
0.05±0.005 bc
Guang Ba Ai
2.8±0.1 de
1.3±0.1 c
0.03±0.005 de
Yin Du Mo Lv Zi
2.3±0.05 fg
1.3±0.05 c
0.03±0.005 de
Da An Qing Pi
2.7±0.20 e
0.7±0.05 ghi
0.03±0.005 e
Gu Ba Chang Jia
1±0.05 k
0.5±0.05 j
0.01±0.005 f
Yong Tai Ge Ling Ma
1.9±0.1 i
1±0.1 ef
0.06±0.005 b
Min-26
1.6±0.15 j
1.2±0.1 cd
0.05±0.005 bc
Shang Huo Ma
0.9±0.05 l
0.6±0.05 ij
0.01±0.005 f
Hun Xuan 19
1.9±0.1 i
1±0.05 f
0.04±0.005 cd
Yun Xiao Hong Pi
2.3±0.1 g
1.2±0.05 cd
0.05±0.01 bc
Yong An Hong Pi
2.2±0.15 h
1.3±0.05 c
0.04±0.005 cd
Ping He Dan Zhu
2.9±0.1 d
1.3±0.05 c
0.05±0.01 bc
He Zi No.8
2.5±0.1 f
0.9±0.1 fg
0.04±0.005 cd
Table 5: Effect of Cu stress on Seedling height, Seeding fresh weight and Seedling dry weight in different varieties of jute. Values in the table is just one harvests ± SD (n =3). Different letters within a column indicate significant difference between the treatments (P‹0.05 or P‹0.01). Relative radiance of plastic filter used different varieties of jute.
Cu however, is an essential nutrients to the plants but require in very small amount. When Cu quantity is increased then the normal range it causes toxicity in plants [33]. Also Cu toxicity effect many physiological process and cause reduction in plant growth and biomass [10]. Excess Cu can affect important physiological processes in plants and cause problems in plant growth and development. Cu taken from the soil must be transported, distributed, and compartmentalized within different tissues and organelles for healthy plant growth and development [34]. On the other hand, excessive Cu is characterized by a reduced plant biomass, leaf chlorosis, inhibited root growth, bronzing, and necrosis. Cu stress to the plants can reduce the yield of plant [35]. Cu taken from the soil must be distributed to the above ground parts of plants for better growth and development of plant [36]. Cu stress may affect the plant growth, root development also to the bronzing and necrosis in the leaves. Cu stress causes a significant reduction in seedling height and biomass. The reduction in the root length and shoot length i.e. total length of seedling may be due to high Cu stress and nutrients imbalance by Cu uptake [24]. Cu stress causes the decrease in the fresh biomass and dry biomass of the seedling. The reduction in the biomass depends upon the seedling height [37]. Same result was showed by [38] when he studied Hydrilla verticillata and also by [39] when he studied sugarcane and also by [13] when he studied B. nivea under Cu stress. Different varieties of jute undergo same Cu stress exhibit different morphological responses are speciesspecific [40,41]. Some varieties show excellent tolerance under Cu stress has ability to survive in the toxic environment while others have lacked this feature. Hence Hong Tie Gu Xuan and C-3 showed good tolerance under Cu stress while Shang Huo Ma and Gu Ba Chang Jia showed poor growth and development.
Conclusion
Based on the findings of the present study, it can be concluded that jute has a considerable potential to cope with Cu stress due to an antioxidative defense mechanism. However, excess Cu toxicity affects the morphological parameters and antioxidants enzymes of jute. While, on the other hand Hong Tie Gu Xuan and C-3 showed resistance against Cu stress while Shang Huo Ma and Gu Ba Chang Jia showed less tolerance against Cu stress. So it can be concluded that, Hong Tie Gu Xuan and C-3 are considered as Cu tolerant varieties can grow a huge biomass under Cu contaminated sited while Shang Huo Ma and Gu Ba Chang Jia are considered as Cu sensitive varieties. Further pot experiments are suggested to perform in the future study.
Acknowledgement
This research was supported by China Agriculture Research System project (CARS-16-E12) and the National Natural Science Foundation of China (31571717).
References
- Nagajyoti PC, Lee KD, Sreekanth TVM. Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett. 2010; 8: 199-216.
- Mengel K, Kirkby EA. Principles of plant nutrition. Ann Bot. 2004; 93: 479- 480.
- Chaignon V, Hinsinger P. Abiotest for evaluating copper bioavailability to plants in a contaminated soil. J Enviro Qual. 2003; 32: 824-833.
- Wuana RA, Okieimen FE. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. 2011.
- Guzel S, Terzi R. Exogenous hydrogen peroxide increases dry matter production, mineral content and level of osmotic solutes in young maize leaves and alleviates deleterious effects of copper stress. Bot Stud. 2013; 54: 26.
- Xu J, Yang L, Wang Z, Dong G, Huang J, Wang Y. Toxicity of copper on rice growth and accumulation of copper in rice grain in copper contaminated soil. Chemosphere. 2006; 62: 602-607.
- Yruela I. Copper in plants. Braz J Plant Physiol. 2005; 17: 145-156.
- Sonmez S, Kaplan M, Sonmez NK, Kaya H, Uz I. High level of copper application to soil and leaves reduce the growth and yield of tomato plants. Scientia Agricola. 2006; 63: 213-218.
- Chand S, Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: an overview. Afr J Microbiol Res. 2009; 3: 981-96.
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford University Press, NewYork. 2007.
- Ku HM, Tan CW, Su YS, Chiu C, Chen CT, Jan FJ. The effect of water deficit and excess copper on proline metabolism in Nicotiana benthamiana. Biol Plant. 2012; 56: 337-343.
- Szabados L, Savoure A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010; 15: 89-97.
- Rehman M, Maqbool Z, Peng DG, Liu LJ. Morpho-physiological traits, antioxidant capacity and phytoextraction of copper by ramie (Boehmeria nivea L.) grown as fodder in copper-contaminated soil. Environ Sci and Poll Re. 2019; 26: 5851-5861.
- Thiruchitrambalam M, Athijayamani A, Sathiyamurth S, Thaheer ASA. A Review on the natural fiber-reinforced polymer composites for the development of roselle fiber-reinforced polyester composite. Journal of Natural Fibers. 2010; 7: 307-323.
- FAOSTAT - Crops, 2014. Query page requires interactive entry in four sections: “Countries”-Select All; “Elements”-Production Quantity; “Items”- Jute; “Years”-2014. Food and Agricultural Organization of United Nations. Economic And Social Department. The Statistical Division. 2017.
- Ahsan N, Lee DG, Lee SH, Kang KY, Lee JJ, Kim PJ, et al. Excess copper induced physiological and proteomic changes in germinating rice seeds. Chemosphere. 2007; 67: 1182-1193.
- Chen CN, Pan SM. Assay of superoxide dismutase activity by combining electrophoresis and densitometry. Bot Bull Acad Sin. 1996; 37:107-111.
- Sakharov IY, Aridilla GB. Variation of peroxidase activity in cacao beans during their ripening, fermentation and drying. Food Chem. 1999; 65: 51-54.
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968; 125: 180-198.
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973; 39: 205-207.
- An YJ. Assessment of comparative toxicities of lead and copper using plant assay. Chemosphere. 2006; 62: 1359-1365.
- Labra M, Gianazza E, Waitt R, Eberini I, Sozzi A, Regondi S, et al. Protein changes in response to potassium dichromate treatments. Chemosphere. 2006; 60: 1234-1244.
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998; 67: 425-479.
- N Nasri, I Saïdi, R Kaddour and M Lachaâl. Effect of Salinity on Germination, Seedling Growth and Acid Phosphatase Activity in Lettuce. Americ J Plant Sci. 2015; 6: 53079-53086.
- Nasr SMH, Parsakhoo A, Skandari S, Gojani HJ, Koohi SKS. Investigation of Salinity Tolerance in Dodonaea viscosa L. J App Bio Sci. 2012; 6: 31-36.
- Quartacci MF, Navari-Izzo F. Water stress and free radical mediated changes in sunflower seedlings. J. Plant Physiol. 1992; 139: 621-625.
- Prochazkova D, Sairam RK, Srivastava GC, Singh DV. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001; 161: 765-771.
- Harinasut P, Poonsopa D, Roengmongkol K, Charoensataporn R. Salinity effects on antioxidant enzymes in mulberry cultivar. Sci Asia. 2003; 29: 109- 113.
- Asish KP, Anath BD. Salt tolerance and salinity effects on plants. A review. Ecotoxicol Environ Saf. 2005; 60: 324-349.
- Rehman M, Liu LJ, Bashir S, Saleem MH, Chen C, Peng DG, et al. Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper contaminated soil. Plant Physio Biochem. 2019; 138: 121-129.
- Sun BY, Kan SH, Zhang YZ, Deng SH, Wu J, Yuan H, et al. Certain antioxidant enzymes and lipid peroxidation of radish (Raphanus sativus l.) as early warning biomarkers of soil copper exposure. J Hazard Mater. 2010; 183: 833-838.
- Chen J, Shafi M, Li S, Wang Y, Wu J, Ye Z, et al. Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens). Sci Rep. 2015; 5: 13554.
- Verma A, Bhatia S. Analysis of some physicochemical parameters and trace metal concentration present in the soil around the area of Pariccha thermal power station in Jhansi, India. Int J Inno Res Sci Eng Technol. 2014; 3: 10482-10488.
- Habiba U, Ali S, Farid M, Shakoor MB, Rizwan M, Ibrahim M, et al. EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ Sci Pollut Res. 2015; 22: 1534-1544.
- Rizwan M, Ali S, Adrees M, Rizvi H, Zia-ur-Rehman M, Hannan F, et al. Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review. Environ Sci Pollut Res. 2016; 23: 17859- 17879.
- Habiba U, Ali S, Farid M, Shakoor MB, Rizwan M, Ibrahim M, et al. EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ Sci Pollut Res. 2015; 22: 1534-1544.
- Raza SH, F Shafiq, M Tahir. Screening of cadmium tolerance in sugarcane using antioxidantive enzymes as a selection criteria. Pak Life Social Sci. 2013; 11: 8-13.
- Nizam MU, Zaman MW, Rahman MM, Jang-Eok-Kim. Phytoremediation Potential of Kenaf (Hibiscus cannabinus L.), Mesta (Hibiscus sabdariffa L.) and Jute (Corchorus capsularis L.) in Arsenic-contaminated soil. Korean J of Envi agri. 2016; 35: 111-120.
- Singh A, CS Kumar, A Agarwal. Physiological study of combined heavy metal stress on Hydrilla verticillata (lf) Royle. Int J Environ Sci. 2012; 2: 2234-2242.
- Lin CC, Kao CH. Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul. 2000; 30: 151-155.
- Yildirim E, Karlidag H, Dursun A. Salt Tolerance of Physalis during Germination and Seedling Growth. Pak J Bot. 2011; 43: 2673-2676.
