Abstract
The flower morphology and breeding system of three species that belong to section Cyphomandrosis (S. confusum, S. glaucophyllum and S. stuckertii) was studied by field experiments and the observation of the pollen tubes growth in the gynoecium, regarding that they are all self-incompatible. In Solanum glaucophyllum flowers with styles of different length were observed some with long styles that protrude from the cone of the androecium and others with short styles that are entirely enclosed by it, these last do not produce fruits, showing andromonoecy. The pollinators of these species are also cited.
Keywords: Solanum; Andromonoecy; Pollination; Breeding system
Introduction
The genus Solanum L., one of the largest in flowering plants, is characterized by a wide diversity both in the vegetative field and in the inflorescences structure [1,2]. On the contrary, the floral syndrome in the genus is almost constant with a particular kind of melitophyly where the pollen is the only reward [3]. The pollen can be actively extracted by means of vibrations of the wing muscles (”buzz pollination“, [4]) or it can be associated to another mechanism, (bellows like), facilitating the reward obtaining by pollinators that do not have this ability to vibrate the anthers and passively receive the pollen on their bodies.
On the other hand, the aspects referred to the different sexual expressions found in Solanum and the evolution of them, are analyzed in works by Hossain, Anderson, Symon, Colemann & Colemann and Anderson & Symon and more recently Diggler studies the role of phenotypic plasticity in the diversification of andromonoecia in Solanum sect [5-9]. Lasiocarpa. The condition of andromonoecy in Solanum, in which the flowers with exerted styles are considered as functionally hermaphroditic and those with short styles are considered as functionally masculine is treated by different authors [10-15]. Quesada-Aguilar, studied the floral morphology and its relationship in the pollination dynamics of Solanum carolinense observing its role in the evolution and maintenance of andromonoecy in the genus [16]. These authors present an interesting summary about this subject, proposing three hypotheses: the formation of masculine flowers as energy economy that can be used in the production of fruits, increase of male fitness through best pollen collection and that of broadening cross-pollination.
Here I study the floral morphology, the breeding system and pollinators of Solanum sect Cyphomandropsis species (S. confusum Morton, S. glaucophyllum Desf. and S. stuckertii Bitter) that grow in different areas of central and northern Argentina, observing the production of fruits in the different types of crosses. It is also regarded pollen tubes germinating in the gynoecium of these plants and the pollinators behavior.
Materials and Methods
Pollination and floral visitors studies were carried out in situ in the three species belonging to Solanum sect. Cyphomandropsis: S. confusum, in the province of Tucumán, Quebrada de los Sosa, S. glaucophyllum in the province of Buenos Aires, La Plata city and S. stuckertii province of Córdoba, Falda de Los Reartes, Argentina. The trials were extended to experimental populations cultivated in the Facultad de Ciencias Naturales y Museo de la Universidad Nacional de La Plata.
The variations of the style length along the top were controlled by daily measurements of each of the flowers that opened in the marked inflorescences, in cultivated plants.
In Solanum glaucophyllum because of the presence of styles with different length; an important number of flowers (150) was collected in the field, at random, to have an approximate value of the frequency of the recorded variation. Histochemical studies in pollen were performed using I/IK (Lugol) to detect the presence of starch, Eosin for proteins and Sudan IV for lipids.
Pollen grains amount per anther was estimated following the technique of [17]. The number of seminal rudiments was counted in sectioned ovaries under a stereoscopic microscope. The pollen/ovule ratio [18] was estimated to obtain indicative data of the reproductive system. To study the reproductive system, in the field, flowers were bagged in paper bags in several blocks, from the day before anthesis. The treatments were the following: 1- Flowers without treatment; 2- Flowers self-pollinated manually; 3- Flowers crossed with pollen from other flowers of the same plant (geitonogamy); 4- Flowers crossed intra-population (xenogamy). For manual pollinations, the pollen was extracted by subjecting the flowers to the vibrations of a tuning fork and transferring the pollen with a brush, taking care to avoid contamination with pollen from another source. The percentage of fruits formed in each case was also obtained. At the laboratory, pollen tube growth were observed with fluorescence microscopy to determine the compatibility mechanisms. The visiting insects were captured. The determinations were made by Alberto Abramovich of the Faculty of Natural Sciences of the UNLP and by Arturo Roig Alsina of the Museum of Natural Sciences ”Bernardino Rivadavia“. The observations on their behavior were made in natural environments at different times of the day.
Results
Inflorescences
The inflorescences of Solanum, sect. Cyphomandropsis influences on pollination, they are pendulous, terminal, pseudolateral and extra axillary. They form scorpioid tops, of simple or double series from a basal peduncle, from which the remaining internodes hang from the top (Figure 1A-F). This is longer than the pedicels, allowing the inflorescence to move away from the foliage and remain exposed to pollinators. The number of flowers per inflorescence varies between 12 and 27 in S. confusum, between 10 and 25 in S. glaucophyllum and between 10 and 20 in S. stuckertii. The anthesis progresses gradually from the base of the inflorescence to the apex. In general, there are only two or three flowers open per day, in each one. In this way, the flowering lasts several days along the top.

Figure 1: Solanum sect Cyphomandropsis inflorescences. A, B, C: S.
confusum. A: General appearance of plant. B-C: Inflorescences. B: Basal
peduncle clearly distant from the main axis. C: Inflorescence with numerous
flowers. D-E: S. stuckertii. F-G: S. glaucophyllum.
Flowers
The three studied species have perfect, actinomorphic, pentameric flowers with gamosepalous and gamopetalous perianth. The corolla has a short tube and a more or less expanded limb with five lobes. In Solanum confusum the calix is cupuliform and the campanulate corolla is brown violet with darker areas in the midrib. During the first day of anthesis, the anthers are violaceous, cryptic, since their color is not contrasted with the corolla. In the following days, they become more yellowish (Figure 2A&B). In Solanum glaucophyllum the calix is cupuliform. The presence of two forms of corolla is observed: mild or lobed. Each of these forms is preserved during the anthesis, being both in the same population and even in the same inflorescence. During the first day the corolla is violaceous with green areas in the center, then it gradually clear up to the whitish blue at the end of the anthesis. The anthers, are citrine yellow (Figure 2C&D). Solanum stuckertii presents campanulate calyx and the corolla is divided almost to the base, with a tube of 2 or 3 mm in length. The petals are white with greenish zones along the middle rib and at the base. The presence of simple hairs is observed in the abaxial apex of the same and on the mid rib on the adaxial surface. During the anthesis, the petals roll up exposing the androecium. The anthers maintain the characteristic yellow color (Figure 2E&F) [19].

Figure 2: Solanum sect Cyphomandropsis flowers. A-B: S. confusum. A:
Lateral view of the flower with two petals removed. C-D: S. glaucophyllum.
C: Frontal view. D: Lateral view of the flower with petals removed. E-F: S.
stuckertii. E: lateral view. F: Frontal view. Some pores open and others
closed.
Ginoecium
The ovary is superior, syncarpic, bicarpelar, bilocular and multiovulated, with axillary placentation. The style is glabrous, cylindrical and solid terminal in the three studied species. It has a transmission tissue in the central zone, with cells with a polygonal section in cross section and vertically elongated. The style culminates in a nailed stigma (Solanum stuckertii and S. confusum) or bilobated (S. glaucophyllum). The receptive surface is represented by a papillose glandular epidermis. These unicellular papillae, uninucleated, of different length and with thin walls, capture the pollen grains with the help of their exudate. According to the classification of Heslop- Harrison [20], it can be included in the WPU type (wet with unicellular papillae).
The number of ovules or seminal rudiments was similar for the three species. They can be consulted in table 1.
Species
DP Rango (X)
DE
Shape DP/DE
No pollen grains/flower
No ovules/
P/O
Solanum adelphum
21,5(23)24,5
17(18)19
Subprolate
1.212.000±312
32±3
37.875
S. glaucophyllum
18(19)20
16(16,5)17
Subprolate
1.138.889±278
45±4
25.309
S. stuckertii
19(19,5)20
17(18)19
Spheroidal
1.300.361±385
56±7
23.22
DP: Polar Diameter
DE: Ecuatorial Diameter
P/O: Pollen Ovule relation a flower.
Table 1: Palynological data, number of ovules and P/O ratio of the three Solanum sect.Cyphomandropsis species.
Style length variations along the inflorescence and in the population
In Solanum glaucophyllum flowers with styles of different length were observed in the same population and in the same inflorescence; some with long styles that protrude from the cone of the androecium and others with short styles that are entirely enclosed by it, not visible from the outside in an open flower (Figure 3A&B). These differences are associated with fertility (see reproductive system). From the anatomical point of view, both floral types are similar. The frequency of flowers with inserted styles is very low, around 15%. In addition, its location at the top does not follow a regular pattern. In the remaining species, the length of the reproductive organs in flowers of the same stage of development does not vary significantly.
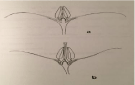
Figure 3: Solanum glaucophyllum flowers with styles of different length. a:
short styles that are entirely enclosed for the androecium. b: flowers with long
styles that protrude from the cone of the androecium.
Pollen morphology, histochemical characters, viability and production
Pollen grains are tricolpate and psilate, medium (between polar diameter 20μm and 17μm ecuatorial diameter) Table 1. Results obtained by histochemical reactions indicate the absence of starch and proteins in the pollen grains of the three analyzed species. A mild reaction with Sudan IV demonstrated the external absence of pollenkitt. However, when observed with Nile Blue, an abundant amount of lipids was found in the cytoplasm [21].
The studies revealed a high degree of pollen fertility in Solanum confusum and S. glaucophyllum. The average values are between 73 and 99%. In the case of Solanum stuckertii, different percentages were observed between the flowers of the first and second day. The flowers of S. stuckertii from the first day of anthesis showed average viability values of 25%, rising to 90% on the second day [21].
The number of pollen grains of the three species analyzed was high, S. confusum: 1.212.000, S. glaucopphyllum: 1.138.000 and S. stuckertii: 1.300.000.
Pollen/ovule relationship
Pollen/ovule relationship is an indicator of the reproductive system. In this case, the observed values (37,875 for Solanum adelphum, 23,220 for S. stuckertii and 25,309 for S. glaucophyllum) were higher than those provided by Cruden [18], placing the 3 species in the category ”xenogamy“ obligated. This data coincides with those obtained experimentally in the field.
Reproduction
Results of the crosses carried out show that the three analyzed species are self-sterile, requiring the presence of biotic vectors (insects) to carry out the pollen exchange and produce fruits with viable seeds.
From the point of view of sexuality, the flowers of Solanum confusum and S. stuckertii are hermaphrodites, with well-developed androecium and gynoecium. On the contrary, S. glaucophyllum presents stylar heterometry (see flowers, gynoecium). Their flowers have a different sexual behavior according to the development of the styles. The flowers with exerted styles are hermaphroditic, having the ability to produce fruit normally. Those that present inserted styles perform staminate and therefore do not produce fruits. This behavior establishes the condition of andromonoecia for S. glaucophyllum. On the other hand, the data obtained from the crossings made in the field confirm the obligatory xenogamy in this taxon. The high percentage of fructification registered indicates a high efficiency in pollination, since the 62.40% of the seminal rudiments, ovules, are fertilized.
There were no significant differences between open and manual pollination Table 2.
Tratamiento
N
Frutos Formados
No
%
Flowers with short styles
Manual Autogamy
60
0
0
Open pollination (clon*)
80
0
0
Open pollination (in the field )
88
49
55,68
Manual Pollination(in the field)
60
35
41,66
Flowers with long styles
Xenogamy (Manual Pollination)
20
0
0
Geitonogamy (Manual Pollination)
20
0
0
Bees pollination in the experimental culture.
N= Number of flowers
Table 2: Breeding System in Solanum glaucophyllum.
In Solanum glaucophyllum and S. stuckertii, in the case of experimental populations with the same genome (clones), only small fruits with aborted seeds are formed as a result of autogamy (selfpollination and geitonogamy, Figure 4 A&B). Contrarily, Solanum confusum does not produce fruit under these conditions.

Figure 4: Normal and abortive fruits. a: Solanum glaucophyllum. b:
S.stuckertii aborive fruits. Valid scale for both photos.
The observation of pollen tubes growing on the stigmas and styles, under fluorescence microscopy, complements the results obtained in the field. The mechanisms of incompatibility, which are visualized with epifluorescence, show that the pollen grains belonging to the same individual (autogamy) can germinate on the stigma. However, its growth does not prosper beyond the first part of the transmission tissue. In figures 5A,B,F&G) and Figure 6A callosa plugs on Solanum confusum belonging to Xenogamy, the clogged pollen tubes, are observed. It is also possible to see the pollen tubes from different individuals growing along the style and its penetration in the seminal rudiments Figure 5C,D&E). In the case of autogamy (Figure 6A), the pollen tubes of S. stuckertii barely germinate on the stigma. In the case of Xenogamy the pollen tubes grow on the stigma and continue through the style (Figure 6B,C&D). The same is observed during Xenogamy in the style and on the stigma in S. glaucophyllum (Figure 7A, B,C&D).
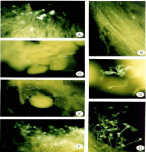
Figure 5: Solanum confusum. A-E: Xenogamy. A: Pollen grains germinating
on stigma. B: Pollen grains growing in the style. C: Ovary. D-E: Pollen 50μm
tubes near the ovule. F-G: Autogamy: Callose obstructing the pollen tubes.
Escales: 2,3,5,7=100μm; 1,4,6=50μm.
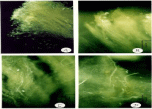
Figure 6: Solanum stuckertii. A: autogamy. Pollen tubes grow only on the
stigma. B: Pollen tubes growing on the stigma. C-D: Pollen tubes in the style.
Escale: 8=100μm, 9,10, 11=50μm.

Figure 7: Solanum glaucophyllum. A-D: Xenogamy. A: Pollen tubes
growing on the stigma. B-C: Growing along the style. D: Datail of A. Escale:
12=100μm, 13,14,15=50μm.
Floral visitors and pollination, Spectrum of visitors, Behavior
The observations indicate that all the species studied have the characteristic biotype of Solanum, corresponding to eutrope flowers, with melitophilia and pollen as a reward.
In Solanum glaucophyllum the bees are taken from the androecium with the jaws and the legs, vibrating each anther separately. Occasionally, insects turn to obtain pollen from different anthers of the same flower. They contact the ventral part of the thorax with the stigma, which emerges 1 or 2 mm from the cone of the anther. The pollen is ejected because of the vibrations produced by the bees and deposited mainly in the sternal region, in the thoracic zone, between the coxas. The larger bees, Bombus atratus, B.belicosus and Xylocopa augusti, hold onto the anthers with their front legs and produce the expulsion of pollen through the pores. This behavior is accompanied by clamping with the jaws, producing a movement of the base towards the apex known as ”milking“ [4]. These bees continue in the same flower, turning and changing anthers, or they are directed to other nearby flowers. By having their bodies covered with pollen, they are taken with their jaws from the anthers, or suspended in the air to perform the grooming movements, collecting and keeping the pollen in the pollen basket. When the insects are small, such as Augochlora sp. and Augochloropsis sp., the dusting area extends to the pleura. In the case of Bombus atratus, it is observed that the stay in the flowers is directly proportional to the amount of pollen available. When anthers begins the dehiscence, or when they are covered with paper bags to exclude the insects and then the protective bag is extracted (thus maintaining the entire pollen reserve), the visits are of longer duration. In this case, the anthesis moment does not influence. Frequently the pollinator moves away from the flower a moment and reiterates several times (5 to 7) his visit to it recognizing, apparently, the amount extracted.
Normally, when anthesis is started, not all pores are open. This causes the pollen to be expelled only by some teak. In this way its delivery is dosed, maintaining throughout the day anthers with its full pollen load for subsequent visits. During the second day, the anthers are more separated and with damaged areas that turn brown, because of the injuries caused by the bees with their jaws. This change in coloration has a negative influence on the frequency of visits. It is observed in the field that the insects omit the flowers.
The first visits at the time of anthesis and the most frequent correspond to Bombus atratus. The earliest collection was recorded at 6 o’clock in the morning, shortly after anthesis. The period of greatest number of visits, coincides between 7.30 and 11 am, although these continue throughout the day, until the fall of the flowers between 8 and 9 pm. In Solanum stuckertii, both, the floral mechanisms and the behavior of the pollinators the patterns are similar to those mentioned. The most frequent visitors of this species in Córdoba province are Bombus opifex and Thygater rubricata (Figure 8). Both bees presented differences between the times of more frequent visits, Bombus opifex is the first species observed in flowers at the time of anthesis (6 am). Visits decrease during the morning, being very scarce at noon. This species returns to be a regular visitor between 4 pm. and 6 pm. On the contrary, the presence of Thygater rubricata is very scarce during the early morning hours. Its frequency increases during the course of the morning registering the highest number of visits (20) between 11 am and 12 am. These bees are directed only to the sector of flowers most exposed to the sun.

Figure 8: A-B: Solanum stuckertii pollination. A: Bombus opifex. B: Thygater
rubricata.
All the visitors produce vibrations of the different anthers and movements of grooming and ”milking“ similar to those described for Solanum glaucophyllum.
The expulsion by vibrations is also observed during the visits of Augochlora sp. and Exomalopsis sp., although due to the small size, these insects usually do not contact the stigma of the flower when they collect pollen. As an unusual visitor, Lonchopria chalybea can be mentioned. All individuals collected from this species visited the same flower, staying twice or triple the time than the other insects at each opportunity.
In Solanum confusum the differences found are associated to the structures and floral morphology. Due that, the anthers remain hidden in the bell-shaped corolla; the direct access of the bees to the androecium is impeded. To be able to vibrate them, the insect must attach itself to the petals with its legs, which in this species and unlike the Solanum glaucophyllum and S. stuckertii, are very fleshy and firm, perhaps adapted to this function. Thus sustained, he manages to place the jaws on the connective and vibrate the androceo, through the spaces that remain between the petals.
The little attraction that Solanum confusum exercise over bees is notorious. Apparently, its flowers are not so showy for insects. During the two years of observation in isolated plants, both in the field, (Quebrada de San Lorenzo, Salta province, with a period of 20 hours of observation adding fragmentary periods) and in culture, did not receive pollinators. In Tafí del Valle (Tucumán province), the only area in which an abundant population was found, only visits of Bombus tucumanensis were recorded in the second year of observation and these were sporadic. It is evident that the attraction is exerted by the effect (perfume and coloration) of several flowers together [19]. The anthers and the perianth, according to the absence of bright colors and contrast zones in the visible and UV segments, do not seem to have a decisive effect on the location of the flowers [19].
Discusion and Conclusion
Each part of the flower can have special participation in one or more functions during reproduction. The organization of the inflorescences and the spatial position of the flowers in the plant are adaptations that increase pollination chances [22]. In the case of Solanum, the inflorescences form a peak with different degree of development. The most elaborate, branched, with many flowers, are considered as relatively primitive by Symon [7]. The species treated here could be included in this group, since they have inflorescences with numerous flowers. In addition, the presence of long axes, which allow to expose the flowers outside the foliage, and contribute to the pollination mode. The same is not true of the pendulous condition of Solanum flowers. According to the experiences of Buchmann and Hurley [23], this character is not obligatory for the functioning of the pollen collection mechanism by means of vibrations. Its function would be linked to the use of androecium by insects, to be held during visits [3].
The gynoecium of the studied species does not present important modifications in comparison with the general characteristics of Solanum. They have wet stigmas with unicellular papillae [20] and an exudate of lipid nature that contributes to the retention of pollen grains. The solid style is common in the Solanaceae, although there are species with a hollow style, with a central stylar channel. Such is the case of Solanum dulcamara L., Brugmansia candida, Datura, Salpiglossis and Bouchetia [24]. During the pollen grains germination in the stigma, the pollen tube grows endotropically through the intercellular spaces in the transmission tissue, as is generally the case with the solid style [25,26].
The percentage of flowers with short styles found in Solanum glaucophyllum is low, however, it would be within the values found for other species of the genus [5]. On the other hand, the amount of pollen grains in these species is always greater than that of those that offer another rewards. This is the typical case of flowers with porous anthers, in which a part of the pollen produced will be used to feed the pollinators and another will contributes to reproduction.
There is, as it is known, a positive correlation between the characteristics of the pollen, the quantity produced and the availability period of the reward. The amount of pollen is increased even more, if the species are xenogamous. The data obtained for the three species in this work are even greater than those provided by Cruden [18].
Regarding the reproductive system, Solanum is undoubtedly one of the best examples to study the different sexual forms, due to the diversity that it presents. Symon [7] and Anderson & Symon [9] cite the presence of andromonoic, hermaphroditic and dioic plants. According to the three studied species, Solanum confusum and S. stuckertii have always hermaphrodite flowers. On the contrary, S. glaucophyllum produces both hermaphrodite and staminate flowers, with reduced, non-functional gynoeciums, presenting andromonoecy. This specie has a low percentage of staminate flowers, so its condition could be classified according to Whalen & Costich [27] as weak andromonoecy. This sexual form is frequent among the species of the subgenus Leptostemonum, in which sec. Cyphomandropsis is treated and is rare in the other subgenus [7,27]. The evolution of andromonoecy in Solanum is explained by the loss of flowers and the reduction of the gynoecium [27], and not by the production of an excess of staminate flowers as some authors assume [9]. This statement is based on the following evidence: the presence of a smaller number of flowers in the inflorescences of species with strong andromonoecy than in those with weak andromonoecy. The presence of a non-functional gynoecium would indicate that the condition of andromonoecy derived directly from hermaphroditism [27]. This statement includes, therefore, S. glaucophyllum. The compatibility system in the genus is also variable, observing both selfcompatible and self-incompatible plants [7,27]. In the three species studied here, self-sterility barriers were found that require xenogamy The presence of wet stigma, with solid style and binuclear pollen, as well as the wide distribution of autoesterility alleles in other members of the Solanaceae [28,29], point out the presence of gametophytic incompatibility in these species.
It could be inferred that incompatibility and andromonoecy could be linked, but data on new species are needed to confirm this hypothesis [7]. The pollen-ovule relationship is used by several authors in the studies on reproductive systems [30,31] however, it is not yet clear what factors influenced the evolution of this relationship [32,16].
Bees diversity with vibratile adaptations to the Solanum flowers is wide [33-35, 3]. However, some bee genera, although they can use the alar muscles for various activities (increase in body temperature, communication), can not vibrate the anthers of pollen species; such is the case of Apis mellifera L. [35]. Most of pollinators found in this work were already cited for other Solanum species. All are able to obtain the reward through vibrations. While Augochlora and especially Augochloropsis were commonly observed collecting pollen, its effectiveness would be lower because of its small body that makes scarce contact with stigma. The species that had low frequency of visits are considered eventual pollinators (Bombus belicosus, Exomalopsis sp.) [36,37].
Acknowledgement
The author is greatly indebted to Dr. Andrea Cocucci who made valuable suggestions and improved the investigation and for his collaboration with the photographs. This paper was written during the development of the PDTS IP 120.
References
- Child A. Studies in Solanum L. and related genera. A provisional conspectus of the genus Cyphomandra Mart.ex Sendtner. Feddes repert. 1984; 95: 283- 298.
- Danert S. Die verzweigung der Solanaceem im Reproduktiven Bereich.Abh. ch.Akad.Wiss.Berlin,Kl.Chem. 1958; 6: 1-183.
- Cocucci AA. Polinización en Solanáceas Neotropicales. Tesis,Universidad Nacional de Córdoba. Argentina. 1988.
- Buchmann SL, Jones CE, Colin LJ. Vibratile Pollination of Solanum douglasii and S. xanti (Solanaceae) in Southern California. Wasmann J Biol. 1989; 81: 289-294.
- Hossain M. Observations on stylar heteromorphism in Solanum torvum s.w.(Solanaceae). Bot. J. Linn. Soc. 1973; 66: 291-301.
- Anderson GJ. Dioecious Solanum species of hermaphroditic origins. An example of a broad convergence. Nature. 1979; 282: 836-838.
- Symon DE. Sex forms in Solanum (Solanaceae) and the role of pollen collecting insects. In Hawkes, Lester & Skekding, Eds. The Biology and taxonomy of Solanaceae. Academic Press. 1979; 385-397.
- Colemann, Colemann JR, Coleman MA. Reproductive biology of an andromonoecious Solanum (S. palinacanthum Dunal). Biotropica. 1982; 14: 69-75.
- Anderson GJ, Symon. Functional Dioecy and Andromonoecy in Solanum Evolution. 1989; 43: 204-219.
- Solomon. Sexual allocation and andromonoecy: resource investment in male and hermaphrodite flowers of Solanum carolinense (Solanaceae). American Journal of Botany. 1986; 73: 1215-1221.
- Emms SK. Andromonoecy in Zigadenus paniculatus (Liliaceae): spatial and temporal patterns of sex allocation. Am. J. Bot. 1993; 80: 914-923.
- Elle E. Sex allocation and reproductive success in the andromonoecious perennial Solanum carolinense (Solanaceae). I. Female success. Am. J. Bot. 1999; 86: 278-286.
- Connolly BA, Anderson GJ. Functional significance of the androecium in staminate and hermaphroditic flowers of Solanum carolinense (Solanaceae). Plant Syst. Evol. 2003; 240: 235-243.
- Huang SQ. Flower dimorphism and the maintenance of andromonoecy in Sagittaria guyanensis ssp. lappula (Alismataceae). New Phytologyst. 2003; 157: 357- 364.
- Cuevas J, Polito VS. The role of staminate flowers in the breeding system of Olea europaea (Oleaceae): an andromonoecious, windpollinated taxon. Ann. Bot. 2004; 93: 547-553.
- Quesada-Aguilar A. 2007. Morfología de la flor, funcionalidad de género y dinámica de los polinizadores en Solanum carolinense: implicaciones para la evolución de la andromonoecia. Tesis de maestría, Universidad de Pittsburgh. (Inédito). 2007; 25: 8-29.
- Ornduff Incompatibility and the pollen economy of Jepsonia parryi. Amer J Bot. 1970; 57: 1036- 1041.
- Cruden R. Pollen-ovule ratios: A conservative indicator of breeding systems in flowering plants. Evolution 1977; 31: 32-46.
- Passarelli L, Bruzzone L. Significance of floral colors and scent in three Solanum sect. Cyphomandropsis species (Solanaceae) with different floral rewards.Aust.J. Bot. 2004; 52: 659-667.
- Heslop Harrison J. Stigma characteristic and Angiosperms taxonomy. Nordic Journal of Botany. 1981; 1: 401-420.
- Passarelli L. Morphology, reserves and pollen viability of some Solanum sect. Cyphomandropsis species. Grana. 1999; 38: 284-288.
- Dafni A. Pollination Ecology. A Practical Approach. Oxford University Press. New york. 1992; 6: 763-778.
- Buchmann SL, Hurley JP. 1978. Biophysical model for buzz pollination in angiosperms. Journal of Theoretical Biology. 1978; 72: 639- 657.
- Hunzike, Subils. Estudios sobre Solanaceae XVIII. Sinopsis taxonómica de Bouchetia. Bol.Soc.Argent.Bot. 1983; 22: 275-295.
- Vasil I. Johri B. The style, stigma and pollen tube. Phytomorphology. 1964; 14: 359- 369.
- Bhatmagar SP, Uma MC. The structure of style and stigma in some tubilorae. Phytomorphology. 1969; 19: 99-108.
- Whalen MD, Costich AE. Andromonoecy in Solanum. In: Dárcy W.Ed. Solanaceae. Biology and Systematics. Columbia University Press. 1986; 284-302.
- Bohs. Crossing studies in Cyphomandra (Solanaceae) and their systematic and evolutionary significance. Am J Bot. 1991; 78: 1683-1693.
- Heslop Harrison Y. Shivanna KR. The Receptive Surface of the Angiosperm Stigma. Annals of Botany. 1977; 41: 1233-1258.
- Queller DC. Pollen ovule ratios and hermaphrodite sexual allocation strategies. Evolution. 1984; 38: 1148-1151.
- Preston RE. Polen-ovule ratios in the Cruciferae. American Journal of Botany. 1986; 73: 1732-1740.
- Mione T, Anderson G. Pollen –Ovule Ratios and Breeding System Evolution in Solanum section Basarthrum (Solanaceae). Amer. J. Bot. 1992; 79: 279- 287.
- Bowers KA. The pollination ecology of Solanum rostratum, Solanaceae). American journal of Botany. 1975; 62: 633-638.
- Linsley, Cazier. Further observations on bees which take pollen from plants of genus Solanum. Pan-Pacific Entomologist. 1963; 39: 1-18.
- Oliveira Filho AT, Oliveira LCA. Floral biology of a popuation of Solanum lycopersicum st. Hil. Solanaceae.Rev. Brasil. Bot. 1988;11: 23-32.
- Coelho CP, Gomesa DC, Guilhermea FA, Souza LF. Reproductive biology of endemic Solanum melissarum Bohs (Solanaceae) and updating of its current geographic distribution as the basis for its conservation in the Brazilian Cerrado. Brazilian Journal of Biology. 2017; 77: 809-819.
- Jones CE, Colin LJ. Vibratile pollination of Solanum douglassii and S.xanti (Solanaceae) in Southern California. Wasmann J.Biol. 1977; 35: 1-25.
