Abstract
Light is the key factor for the better growth and development of plant that directly fluctuates biosynthesis of many secondary metabolites. Moreover, light is most important abiotic factor that play very important role in photosynthesis and morphogenesis in the plants body. Therefore, it is very necessary to provide appropriate amount of light for a healthy and normal growth of plant body. The aim of the present study to evaluate the growth and antioxidant capacity of rapeseed using White Light (WL), Dark Red Light (DL), Mixed (red + dark red lights) (ML), Red Light (RL), Blue Light (BL) and Orange Light (OL). Artificial Light Emitting Diodes (LEDs) were used in this experiment in the glass house environment. The lights were provided with LEDs with the peak wavelength of WL 390nm, DL 670nm, ML 650nm, RL 660nm, BL 450nm and OL 610nm. The results revealed that DL, ML and RL promoted plant growth, photosynthetic pigments while BL and OL undergoes high stress and reduced plant growth and photosynthetic pigments when compared with WL. Moreover, the antioxidants enzymes i.e. Superoxidase Dismutase (SOD), Peroxidase (POD) and the contents of Malondialdehyde (MDA), proline and total soluble protein also play very important role when plant undergoes any environmental stress. The results revealed that BL and OL induced high values of SOD, POD, MDA, proline and total soluble protein undergoes high level of stress while DL, ML and RL showed low light stress. Based on the present findings, it can be concluded that OL and BL reduced growth and photosynthetic pigments in rapeseed seedlings while DL, ML and RL promote plant growth and photosynthetic pigments and thus can be used as industrial scale to fulfil market demand of rapeseed oil.
Keywords: Rapeseed seedlings (Brassica Napus L.); Light emitting diodes; Photosynthetic pigments; Growth; antioxidants; Proline; Total soluble protein
Introduction
Light is one of the key elicitors that is the most important environmental factor and its quality plays a fundamental role in photosynthesis and morphogenesis that alters plant architectural development [1]. Only under appropriate amount of light plant can show very good growth and photosynthetic pigments. Any change in the intensity to light due to environmental problems may strongly affect plant’s anatomical, physiological, morphological and biochemical properties of the leaves [2,3]. Plant responses differently to different quality of lights. Green plants make their own food using light intensity called photosynthesis also trigger by light quality [4]. Traditionally many artificial light sources were used like sodium lamps, incandescent lamps, fluorescent lamps, and metal halide lamps [5]. These artificial sources of lights have poor quality of light for plant growth and development and consuming high voltages of electricity [6]. Therefore, there is a need for an efficient source of light having a good quality of light also consuming very few costs. Light-Emitting Diodes (LEDs) are solid-state semiconductors that produce an arrow spectrum and non-coherent light and are much more efficient than any glass-envelope lamp [7]. It is a promising technology for the greenhouse industry that has potential benefits over traditional lighting systems due to their specific wavelength, smaller size, durability, long lifetime, and cool emitting surfaces [8]. LEDs can be adjusted to emit light in very specific parts of the spectrum [9]. For example, chlorophyll absorbs mainly in the blue, green, and red parts of the spectrum but absorbs a very little in the orange and yellow. So, light should be produced only in these parts of the spectrum which is possible with the use of LEDs. These characteristics permit the use of LEDs for plants with particular spectral ranges and it confirms the precise manipulation of spectral quality, intensity, and independent control of spectral ranges [10]. Moreover, different colors of lights have different wavelengths like red (600-700 nm), blue (400-500 nm) and orange (590-620 nm) [11]. These spectra of light are the most important spectra due to their important function in plant development. Red light can excite the biologically inactive phytochrome Pfr form into a biologically active Pfr form, which has maximum absorbance in Far-Red (FR) light [11]. Blue light can stimulate the activities of cryptochrome and phototropin [12,13]. While orange light reduced plant growth and development and not only affects the morphology and physiology but it also regulates the stomatal conductance and development of plants [5,14].
When plants undergo some environmental stress antioxidants come into play to reduce the environmental stress [15]. Stress conditions causes the generation of Reactive Oxygen Species (ROS) in the tissues of the plants which then scavenging by the action of enzymatic antioxidants like Superoxidase Dismutase (SOD), Peroxidase (POD) and non-enzymatic antioxidants like Malondialdehyde (MDA) and proline [16]. Report showed that when plants subjected to the stress condition, there was accumulation of proline contents in the leaves of the plants [17]. Mechanism of high amount of proline accumulation in the leaves of the plants is related to increased synthesis, decreased catabolism, or increased degradation of proteins [18]. Membrane destabilization in terms of higher Malondialdehyde (MDA) contents in many species has also been reported in response to environmental stress [19]. Moreover, expression of antioxidative enzymes such as SOD and POD also plays very important role in reducing the environmental stress [20]. The SOD catalyzes the dismutation of superoxide to H2O2 and molecular oxygen whereas POD decomposes H2O2 by oxidation of cosubstrates [21,22].
Rapeseed (Brassica napus L.) is an important oil crop, but growing this crop on an industrial scale may affect food security in China [23]. Whereas plants cultivated in the natural environment can be exposed to unfavorable growth conditions, controllable artificial facilities can provide optimal conditions for cultivating plants, including rapeseed. Rapeseed is the second largest oil crop in the world, and China ranks second in the world in the production of rapeseed [24]. Moreover, the “double-low” (low erucic acid and low glucosinolate) rapeseed seedling is also a popular vegetable, which can be eaten fresh or produced as a dried vegetable for export. More than 60 million tons of rapeseed is produced per annum [25]. In China, the main producing area of rapeseed is the Yangtze River basin, which contributes more than 85% to the total production [26]. Rapeseed is grown for the production of animal feed, edible vegetable oils and biodiesels. Moreover, rapeseed is second-leading source of protein meal after soya bean [27].
In the future, knowledge of appropriate artificial cultivation conditions will be important for more applications, for example, space planting. To optimize plant growth in artificial facilities, it is important to establish suitable environmental conditions, especially light intensity. In this study, we evaluated the growth and development of rapeseed seedlings grown under different LEDs. Moreover, the effects of different light intensities on the growth and development of rapeseed seedling grown under different colors of lights. Growth parameters, photosynthetic pigments and antioxidant capacity was also measured in this study. Based on the results, it can be speculated about the self-adjustment ability and regulation mechanisms related to rapeseed seedlings grown under different light intensities. Additionally, this research will aid in designing the appropriate light environment to promote the growth of rapeseed seedlings and to provide a theoretical basis for standardized cultivation of oil seed plants.
Materials and Methods
Plant growth conditions
A petri dish experiment was conducted under glasshouse environment with different artificial lights with day and night temperature range outside (2-5oC) inside chamber (20-30oC) and day/night humidity of 80/90 % in Huazhong Agricultural University Wuhan, China (114.20'E, 30.28'N; 50 m above sea level) during October 2018. Seeds were surface sterilized with 0.1% HgCl2 for the prevention of surface fungal/bacterial contamination and ten seeds in each petridish, which remain under LED for 21 days. The seeds of ‘Huaza No. 5’ were used on filter paper (released from different research institute of Hubei Province, China). The wavelengths of these lights are WL (390nm), DL (670nm), ML (660nm), RL (650nm), BL (450nm) and OL (610nm) as shown in (Figure 1). Petri dishes kept under LEDs for 12 h and rest of the time LEDs were off. The experiment was conducted in completely randomized design with three replicates for each treatment. Intensity of light under different LEDs were recorded by (LI6400, Li-Cor, Nebraska, USA). At 21 Days After Sowing (DAS) seedlings were harvest for different morphological parameters (total height, root length, shoot length and plant fresh weigh) and enzymatic study (SOD, POD, MDA, proline and total soluble protein). All chemicals used were of excellent quality and taken from Sinopharm Chemical Reagent Co., Ltd.
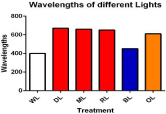
Figure 1: Relative wavelengths of WL (White Light) DL (Dark Light) ML (Mix
Light) RL (Red Light) BL (Blue Light) and OL (Orange Light) spectral of light.
Sampling and data collection
The plants were harvest at 21 DAS for different growth attributes. Plants in each treatment was harvested and separated into roots and shoots for growth and morphology traits. Plant height was defined as the height of the plant from the surface of petri dish to the tip of the uppermost shoot. Plant fresh weight was measured by measuring the weight of plant with the help of weighing balance machine. Plants were separate into roots and shoots then root length and shoot length were also measured. After measuring the growth parameters leaves were washed with distilled water and kept in liquid nitrogen then stored at -80oC for determination of chlorophyll and antioxidants.
Chlorophyll contents
Chlorophyll concentrations were calculated by the standard method of Arnon [28] and expressed in mg g-1 Fresh Weight (FW).
Antioxidants enzymatic activities
The method for measuring the enzymatic-activity of SOD was described by Chen and Pan [29] and expressed as U g-1 FW. The method for measuring the enzymatic-activity of POD was described by Sakharov and Aridilla [30] and expressed as U g-1 FW min-1.
Lipid peroxidation and proline content
The method described the concentration of lipid peroxidation was presented by Heath and Packer [31] and expressed as nmol g-1 FW. Proline contents was measured by the procedure of Bates et al [32] and expressed as μmol g-1 FW.
Statistical analysis
All the results were given as arithmetic means with standard deviations except otherwise defined. Data were tested with one-way ANOVA, followed by HSD tests using Statistix 8.1. The significance level was set at P‹0.05 or P‹0.01. Graphical presentation was carried out using Graph Pad Prism 6.
Results and Discussion
Effect of different colors of light on plant growth
Light is an important component in the growth of a plant besides water and oxygen. Plants are entirely dependent on light energy to prepare their own food, which they may use to continue their life processes, including growth and development [33]. Specifically, changes in light quality due to the spectral properties of tissue pigments strongly affect plant anatomical, physiological, morphological, and biochemical parameters of leaves [34]. Research under varying light conditions suggested that plants responses to light quality are species specific [35-37]. The visible light spectrum radiates light in red, orange, yellow, green, blue, indigo, and violet color. Different color wavelengths have a specific role in plant growth and development. Plants use wavelengths between 400 and 700 nanometers (nm) for their photosynthetic activities, which are responsible for all the energy needs of the plant [38]. In this study, we tried to investigate the effects of light quality White Light (WL), Dark Red Light (DL), Mixed (red + dark red lights) (ML), Red Light (RL), Blue Light (BL) and Orange Light (OL) on growth, photosynthesis, and radical oxygen species production of rapeseed seedlings to determine the ideal light emission spectrum for optimal plant growth. In the present study, the growth parameters like plant height, root length, shoot length and plant fresh weigh were positively influenced by DL, ML and RL and reduced by BL and OL when compared with WL. In the present study, growth in terms height of the plant (cm) as well as other morphological parameters likes root length (cm), shoot length (cm) and total plant fresh weigh (g) (Table 1). Total fresh weigh of plant was decreased by 74% (P‹0.05) under OL and raised up by 87% (P‹0.05) under RL compared with WL treated plants. Plant height was increased by 77% under exposure to RL whereas decreased by 50% (P‹0.05) and 69% (P‹0.05) under OL and BL respectively, as compared with WL while plant height was increased by 88% under exposure to RL whereas decreased by 76% (P‹0.05) and 87% (P‹0.05) under OL and BL respectively, when compared with WL. Root length and shoot length shown significant increase when plants were grown under RL, ML and DL whereas BL and OL reduced these traits. In some plants, a system of sensory photoreceptors has evolved to perceive changes in the ambient light environment [39]. Thus, varying light quality produces different morphogenetic responses in plants. In the present study, BL and OL significantly reduced seedling growth and biomass in rapeseed while DL, ML and RL seemed to improve plant growth reflected in higher total biomass and seedling height when compared with WL. Similar results were observed in studies of Pelargonium, which found that longer wavelengths of light promoted plant growth and stem elongation and shorter wavelengths prevented stem elongation [40]. BL and OL as a radiation-induced stress can act to lower growth in plants whereas RL may increase plant growth and biomass [41] [42]. Although various reports confirmed the morphological and physiological effects of light quality, but these responses vary considerably depending upon plant species. In this view, it is necessary to use proper light system to meet different objectives including biomass, promotion or inhibition of shoot and root formation and production of metabolites of interest [40,43].
Treatment
Plant height
Root length
Shoot length
Total Weight
WL
6.50±0.12 b
1.53±0.03 b
4.97±0.12 b
2.70±0.06 b
DL
8.60±0.06 a
1.80±0.06 a
6.80±0.06 a
3.20±0.06 a
ML
8.36±0.14 a
1.73±0.03 ab
6.63±0.09 a
3.10±0.06 a
RL
8.42±0.12 a
1.80±0.06 a
6.67±0.09 a
3.10±0.06 a
BL
4.52±0.12 c
1.27±0.03 c
3.30±0.06 c
2.63±0.03 b
OL
3.38±0.15 d
0.93±0.03 d
2.50±0.06 d
2.00±0.06 d
Values in the table is just one harvests ± SD (n=3). Different letters within a column indicate significant difference between the treatments (P<0.05 or P<0.01). Relative radiance of plastic filter used: WL white light, DL (Dark Light), ML (Mix Light), RL (Red Light), BL (Blue Light) and OL (Orange Light).
Table 1: Effect of different colors of light on plant height, root length, shoot length and total weight on rapeseed seedlings.
Effect of different lights on chlorophyll contents
Photosynthetic pigments of plants like chlorophyll-a, chlorophyll-b, carotenoid, absorb different spectra of light which play a significant role in the photosynthesis [4]. Chlorophyll is a subtle reflection of primary reactions of photosynthesis. Light has a direct relationship with chlorophyll content and its fluctuation sometimes enhance secondary metabolites production [44]. Difference in the levels of chlorophyll and carotenoid was evaluated in rapeseed under different light quality treatments are presented in (Table 2). Contents of total chlorophyll and carotenoid were raised up by 83% (P‹0.05) and 79% (P‹0.05) respectively under RL when compared with WL but the contents of total chlorophyll and carotenoid were reduced by 93% (P‹0.05) and 84% (P‹0.05) respectively under OL when compared with WL. Chlorophyll and carotenoids are important biologic pigments, which are utilized for photosynthetic conversion of inorganic molecules or ions to organic bio-molecules. In the present study, RL significantly increased contents of chlorophyll and carotenoid, whereas BL and OL resulted in a decrease in contents of chlorophyll and carotenoid. Decreases in chlorophyll concentrations in BL and OL could indicate excessive-irradiance-induced damage to pigments [45]. Thus, excessive light could induce damage to pigments under BL and YL.
Treatment
Chlorophyll A
Chlorophyll B
Total Chlorophyll
Carotenoids
WL
1.56±0.02 ab
0.48±0.02 ab
2±0.05 bc
0.44±0.1 bc
DL
1.7±0.06 a
0.61±0.02 a
2.2±0.1 ab
0.65±0.2 a
ML
1.65±0.05 a
0.53±0.01 a
2.2±0.02 ab
0.70±0.1 a
RL
1.67±0.07 a
0.60±0.02 a
2.4±0.3 a
0.53±0.2 ab
BL
1.47±0.04 b
0.40±0.02 b
1.88±0.04 c
0.35±0.02 c
OL
1.49±0.03 b
0.37±0.06 b
1.86±0.04 c
0.37±0.02 d
Values in the table is just one harvests ± SD (n =3). Different letters within a column indicate significant difference between the treatments (P<0.05 or P<0.01). Relative radiance of plastic filter used: WL white light, DL (Dark Light), ML (Mix Light), RL (Red Light), BL (Blue Light) and OL (Orange Light).
Table 2: Effect of different colors of light on Chlorophyll A, Chlorophyll B, Total Chlorophyll and Carotenoids on rapeseed seedlings.
Effect of different lights on antioxidants, malondialdehyde and proline
ROS can be generated by the direct transfer of the excitation energy from chlorophyll to produce singlet oxygen or by oxygen reduction in the Mehler reaction in the chloroplasts [46], which leads to lipid peroxidation and then membrane damage, as reflected in high levels of MDA and REC. ROS, such as O2-, OH, and H2O2, may oxidize proteins, lipids, and nucleic acids which results in abnormalities at the cellular level [47]. In the present study, the activities of antioxidant enzymes i.e., SOD and POD were affected by differential light quality (Figure 2). The enzymatic- activity of SOD raised up by 87% and 86% (P‹0.05) under OL and BL respectively, when compared with WL. The activity of SOD decreased by 84% (P‹0.05) under RL when compared with WL. Light quality and intensity also play a very important role in regulation the activity of POD enzyme. The enzymatic-activity of POD raised up by 84% and 83% (P‹0.05) under OL and BL respectively, when compared with WL. The activity of POD decreased by 88% (P‹0.05) under RL when compared with WL (Figure 2). Thus, OL and BL trigger higher antioxidant activities in rapeseed whereas RL, ML and DL reduced it. In the same way, OL and BL causes severe damage to the membrane of lipids in this experiments when compared with WL. Proline contents were raised by 97% and 96% (P‹0.05) by OL and BL respectively, when compared with WL in petri dish experiment whereas the contents of proline was reduced by 92% (P‹0.05) under RL when compared with WL (Figure 3). Malondialdehyde (MDA) contents were raised by 86% and 80% (P‹0.05) by OL and BL respectively, when compared with WL whereas the contents of MDA were reduced by 65% (P‹0.05) under RL when compared with WL (Figure 3). However, the contents of total soluble protein were raised by 84% and 85% (P‹0.05) by OL and BL respectively, when compared with WL. The contents of total soluble protein were reduced by 93% (P‹0.05) under RL when compared with WL. The highest total protein content of (784nmlog-1) was observed under OL However, the lowest value (617nmlog-1) was observed in the DL. Feng et al. reported that when plants grown under OL and BL they induced high activity of SOD and POD and showed more contents of MDA and proline in the leaves [48]. In the present study, plants under BL and OL light not only had higher contents of O2- and H2O2,but also maintained higher values of MDA and proline than those under WL, indicating that oxidative damage to lipid membranes occurred in plants exposed to BL and OL. The interrelationship of colored lights and POD production in strawberry plantlets; they showed that red light decreased POD along with other antioxidant enzymes [49]. Similarly, a parallel correlation between elevated (MDA) content and increased activity of POD was reported by Cai et al. [50]. The reason might be that peroxidase activity is greater under oxidative stress, in order to remove toxic hydrogen peroxide with high MDA formation due to lipid peroxidation. Thus BL and OL undergoes high stress induced high enzymatic activities, proline, MDA and total soluble protein.
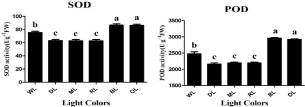
Figure 2: Influence of different light quality treatments on SOD (a) and POD
(b) in rapeseed seedlings. Relative radiance of plastic filter used: WL white
light, DL (Dark Light), ML (Mix Light), RL (Red Light), BL (Blue Light) and OL
(Orange Light). Letters indicate statistical differences (p≤0.05) according to
an HSD test. n=3.
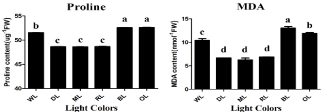
Figure 3: Influence of different light quality treatments on Proline (a) and
MDA (b) in rapeseed seedlings. Relative radiance of plastic filter used:
WL white light, DL (Dark Light), ML (Mix Light), RL (Red Light), BL (Blue
Light) and OL (Orange Light). Letters indicate statistical differences (p=0.05)
according to an HSD test. n=3.
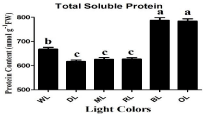
Figure 4: Influence of different light quality treatments on Total Soluble
Protein in rapeseed seedlings. Relative radiance of plastic filter used: WL
white light, DL (Dark Light), ML (Mix Light), RL (Red Light), BL (Blue Light)
and OL (Orange Light). Letters indicate statistical differences (p=0.05)
according to an HSD test. n=3.
Conclusion
On the basis of the present findings, it can be concluded that RL promoted plant growth by activating photosynthetic processes and reducing ROS accumulation in rapeseed seedlings whereas, BL and OL reduced plant growth and photosynthesis, produced higher contents of O2- and H2O2 and greater values of MDA and proline. This was associated with an improvement in the photochemical efficiency and antioxidant system of plants. To our knowledge, this study is the helps to uncover the morphological, physiological, and enzymatic mechanism of responses to light quality for rapeseed seedlings, and provide a theoretical basis for standardized cultivation of rapeseed seedlings on industrial scale for betterment of oil seed quality.
Acknowledgement
This research was supported by the National Natural Science Foundation of China (31571717) and China Agriculture Research System project (CARS-16-E12). Author Contributions Compliance with Ethical Standards.
References
- Kim SJ, Hahn EJ, Heo JW, Paek KY. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sc. Hortic. 2004; 101: 143-151.
- Haliapas S, Yupsanis TA, Syros TD, Kofidis G, Economou AS. Petunia 9 hybrida during transition to flowering as affected by light intensity and quality treatments. Acta Physiol Plant. 2008; 30: 807-815.
- Fan XX, Xu ZG, Liu XY, Tang CM, Wang LW, Han XL. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci Hortic. 2013; 153: 50-55.
- Rehman M, Ullah S, Bao Y, Wang B, Peng D, Liu L. Light-emitting diodes: whether an efficient source of light for indoor plants? Environ Sci Pollut Res. 2017; 24: 24743-24752.
- Morrow RC. LED lighting in horticulture. Hortscience. 2008; 43:1947-1950.
- Gupta SD, Jatothu B. Fundamentals and applications of light emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. 2013; 7: 211-220.
- Bourget CM. An introduction to light-emitting diodes. Hort science. 2008; 43: 1944-1946.
- Mitchell CA, Both A, Bourget CM, Kuboto C, Lopez RG, Morrow RC, et al. LEDs: the future of greenhouse lighting. Chronica Hortic. 2012; 55: 6-12.
- Singh D, Basu C, Meinhardt-Wollweber M, Roth B. LEDs for energy efficient greenhouse lighting. Renew Sust Energ Rev. 2015; 49: 139-147.
- Folta KM, Koss L, McMorrow R, Kim H-H, Kenitz JD, Wheeler R, et al. Design and fabrication of LED-based light arrays for plant research. BMC-Plant Biol. 2005; 5: 17-28.
- Li JG, Li G, Wang HY, Deng XW. Phytochrome signaling mechanisms. Arabidopsis Book. 2011; 3: e0148.
- Inoue S-I, Takemiya A, Shimazaki K-I. Phototropin signaling and stomatal opening as a model case. Curr Opin Plant Biol. 2010; 13: 587-593.
- Yu X, Liu H, Klejnot J, Lin C. The cryptochrome blue light receptors. Arabidopsis Book. 2010; 8: e0135.
- Folta KM. Green light stimulates early stem elongation, antagonizing lightmediated growth inhibition. Plant Physiol. 2010; 135: 1407-1416.
- Prochazkova D, Sairam RK, Srivastava GC, Singh DV. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001; 161: 765-771.
- Mastropasqua L, Borraccino G, Bianco L, Paciolla C. Light qualities and dose influence ascorbate pool size in detached oat leaves. Plant Sci. 2012; 183: 57-64.
- Ku HM, Tan CW, Su YS, Chiu C, Chen CT, Jan FJ. The effect of water deficit and excess copper on proline metabolism in Nicotiana benthamiana. Biol Plant. 2012; 56: 337-343.
- Kishor K, Sangam PB, Amrutha S, Sri Laxmi RN, Naidu P, Rao KR, et al. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr Sci. 2005; 88: 424-438.
- Rehman M, Maqbool Z, Peng D, Liu L. Morpho-physiological traits, antioxidant capacity and phytoextraction of copper by ramie (Boehmeria nivea L.) grown as fodder in copper-contaminated soil. Environ Sci Pollut R. 2019; 26: 5851- 5861.
- Rehman M, Liu L, Bashir S, Saleem MH, Chen C, Peng D, et al. Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper contaminated soil. Plant Physio Biochem. 2019; 138: 121-129.
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford University Press. NewYork. 2007; 888.
- Zhao S, Liu Q, Qi Y, Duo L. Responses of root growth and protective enzymes to copper stress in Turfgrass. Acta Biol Cracov Ser Bot. 2010; 52: 7-11.
- Wang L, Feng ZC. Evaluation of rape industry chain and the security situation estimation. Industrial Economy. 2013; 12: 58-67.
- Li H, Tang C, Xu Z. The efects of different light qualities on rapeseed (Brassica napus L.) plantlet growth and morphogenesis in vitro. Sci Hort Amsterdam. 2013; 150: 117-124.
- Food and Agricultural Organization (FAO). Land and plant nutrition management service. 2011.
- Li Z, Wu BJ, Lu GY, Chen Y, Zou CS, Zhang XK. Differences in physiological responses of brassica napus genotypes under water stress during seedling stage. J Oil Crops Sci. 2012; 34: 033-039.
- Kamran M, Malik Z, Parveen A, Huang L, Riaz M, Bashir S, et al. Ameliorative Effects of Biochar on Rapeseed (Brassica napus L.) Growth and Heavy Metal Immobilization in Soil Irrigated with Untreated Wastewater. J of Plant Growth. 2019.
- Arnon DT. Copper enzyme in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949; 24: 1-15.
- Chen CN, Pan SM. Assay of superoxide dismutase activity by combining electrophoresis and densitometry. Bot Bull Acad Sin. 1996; 37: 107-111.
- Sakharov IY, Aridilla GB. Variation of peroxidase activity in cacao beans during their ripening, fermentation and drying. Food Chem. 1999; 65: 51-54.
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968; 125: 180-198.
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973; 39: 205-207.
- Fukuda N, Fujita M, Ohta Y, Sase S, Nishimura S, Ezura H. Directional blue light irradiation triggers epidermal cell elongation of abaxial side resulting in inhibition of leaf epinasty in geranium under red light condition. Sci Hortic. 2008; 115: 176-182.
- Shimizu H, Saito Y, Nakashima H, Miyasaka J, Ohdoi K. Light environment optimization for lettuce growth in plant factory. In: Vol. 18 Proceedings of the 18th IFAC World Congress. 2011; 605-609.
- Cope KR, Bugbee B. Spectral effects of three types of white light-emitting diodes on plant growth and development: absolute versus relative amounts of blue light. HortScience. 2013; 48: 504-509.
- Hogewoning SW, Maljaars H, Harbinson J. The acclimation of photosynthesis in cucumber leaves to different ratios of red and blue light. Photosynth Res. 2007; 91: 287-288.
- Nanya K, Ishigami Y, Hikosaka S, Goto E. Effects of blue and red light on stem elongation and flowering of tomato seedlings. Acta Hortic. 2012; 956: 261-266.
- Kami C, Lorrain S, Homitschek P, Fankhauser C. Chapter two: light regulated plant growth and development. Plant Dev. 2010; 91: 29-66.
- Gyula P, Schafer E, Nagy F. Light perception and signalling in higher plants. Curr Opin Plant Boil. 2003; 6: 446-452.
- Appelgren M. Effects of light quality on stem elongation of Pelargonium in vitro. Sci Hortic. 1991; 45: 345-351.
- Wellmann E. UV radiation in photomorphogenesis. Photomorphogenesis. Springer, Berlin Heidelberg. 1983; 745-756.
- Li Q, Kubota C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ Exp Bot. 2009; 67: 59-64.
- MHM Silva, PC Debergh. The effect of light quality on the morphogenesis of in vitro cultures of Azorina vidalii (Wats.) Feer, Plant Cell Tiss. Org. Cult. 1997; 51; 187-193.
- Ahmad N, Abbasi BH, Fazal H, Khan MA, Afridi MS. Effects of reverse photoperiod on in vitro regeneration and piperine production in Piper nigrum. CR Biol. 2014; 337: 19-28.
- Shao QS, Wang HZ, Guo HP, Zhou AC, Huang YQ, Sun YL, et al. Effects of shade treatments on photosynthetic characteristics, chloroplast ultrastructure, and physiology of Anoectochilus roxburghii. PLoS One. 2014; 9: 85996.
- Stepien P, Klobus G. Antioxidant defense in the leaves of C3 and C4 plants under salinity stress. Physiol Plant. 2005; 125: 31-40.
- Prochazkova D, Sairam RK, Srivastava GC, Singh DV. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001; 161: 765-771.
- Feng JC, Zhang YJ, Zhang QJ, Li JD, Bi HT, Wu YX. Effects of different light quality on physiological and biochemical indexes in Camptoeca acuminata under low light condition. Nonwood Forest Res. 2008; 26: 1-7.
- Qin Y, Zhang S, Syed A, Zhang L, Qin Q, Chen K. Regeneration mechanism of Toyonoka strawberry under different color plastic films. Plant Sci. 2005; 168: 1425-1431.
- Cai X, Kang XY. In vitro tetraploid induction from leaf explants of Populus pseudosimonii Kitag. Plant Cell Rep. 2011; 30: 1771-1778.
