
Special Article - Microextractions & Molecular Imprinted Polymers for Sample Preparation
Austin J Anal Pharm Chem. 2015;2(3): 1042.
Dispersive Liquid-liquid Microextraction-injector Port Silylation: A Viable Option for the Analysis of Polar Analytes using Gas Chromatography-Mass Spectrometry
Rajeev Jain1,5, Anu Kumar², Yogeshwer Shukla3,4 and Mohana Krishna Reddy Mudiam1,4*
1Analytical Chemistry Section, CSIR-Indian Institute of Toxicology Research, M G Marg, Lucknow, India
2CSIRO Land and Water, Private Mail Bag 2, Glen Osmond, South Australia 5064, Australia
3Proteomics Laboratory, CSIR-Indian Institute of Toxicology Research, M G Marg, Lucknow, India
4Academy of Scientific and Innovative Research, Council of Scientific and Industrial Research, Lucknow, India
5Central Forensic Science Laboratory, Directorate of Forensic Science Services, Ministry of Home Affairs, Govt of India, Lachit Borphukan Path, Guwahati-781012, India
*Corresponding author: Mohana Krishna Reddy Mudiam, Analytical Chemistry Section, CSIR-Indian Institute of Toxicology Research, M G Marg, Lucknow,India.
Received: April 16, 2015; Accepted: May 26, 2015; Published: May 29, 2015
Abstract
Analysis of analytes with polar functional groups using gas chromatographymass spectrometry pose challenges due to adsorption of these analytes on the active sites of injector port and capillary column. These can be overcome by performing derivatization. An attempt has been made to review the literature to understand the injector port derivatization (particularly silylation) coupling with dispersive liquid-liquid microextraction for the analysis of polar analytes and its use in the analysis of chemical analytes containing polar functional groups.
Keywords: Injector port silylation; GC-MS; DLLME; Injector port derivatization; BSTFA
Introduction
Development of modern sample preparation techniques is aimed to focus on the use of zero or minimum amount of toxic solvents for extraction and to reduce the cost and time of analysis in the whole extraction procedure. In recent years, development of microextraction techniques such as solid-phase microextraction (SPME), single drop microextraction (SDME), and dispersive liquidliquid microextraction (DLLME) etc has attracted a great promise for effective sample preparation techniques. Conventional gas chromatography (GC) or gas chromatography-mass spectrometry (GC-MS) is not an ideal choice to study polar, hydrophilic and nonvolatile compounds as these compounds are well adsorbed on the active sites of injector port and column, additionally intra-molecular hydrogen bonding also interferes with the analysis of polar analytes by GC. This problem can be overcome by derivatizing polar analytes with a suitable derivatizing reagent. Derivatization increases the volatility, detectability and thermal stability of polar compounds. Out of the derivatization reagents reported, silylation is the most preferred derivatization and it has found wide applications for the analysis of polar analytes using GC or GC-MS analysis [1].
Injection Port Silylation (IPS)
Silylation is the most widely used derivatization method for the conversion of polar analytes into non-polar derivatives [1]. However, a conventional silylation which is performed outside the GC-MS injection port in a reaction vessel requires high temperature (~60– 80°C), longer reaction time (~30–120 min) and large volume of toxic solvents/reagents. In order to overcome these limitations for rapid, sensitive and reproducible methods, Rasmussen has introduced a technique called injection port silylation (IPS) which is an online derivatization technique [2]. It is a gaseous phase reaction between a silylating reagent and polar analytes which occurs inside the hot GC or GC-MS injection port. Basically, IPS is a type of injection port derivatization (IPD), which also includes derivatization of polar analytes with ion-pair reagents such as tetra alkyl ammonium salts (TAA) such as tetrabutylammonium hydrogen sulphate (TBAHS), tetrabutylammonium chloride (TBAC) and tetrabutylammonium hydroxide (TBAH) [3-5]. In solution form, the TAA forms an ionpair complex with analytes containing carboxylic or sulfonic acid groups which upon the introduction in hot GC-MS injection port forms an ester with polar analyte and tertiary amines as by-products. However, the major constraint of alkylation with TAA is that, only acidic functional groups can be derivatized.
In contrast to IPD with TAA, IPS overcomes the aforesaid limitations and can derivatize polar functional groups such as –OH, -NH2, -COOH, -SH. Additionally, IPS also reduces the possibilities of degradation of derivatives as their exposure to moisture sensitive conditions is negligible. IPS has overcome the major problems associated with traditional in-vial silylation. Extra experimental apparatus such as the heater and reaction vials are not required for IPS derivatization as reagent and analytes are simultaneously or one by one injected inside the GC injection port. In addition, the amount of reagent required for derivatization and sample is greatly reduced from microliters to nanoliters. The reaction efficiency of on-line derivatization is also improved when compared to off-line derivatization which subsequently enhances the detector sensitivity and accuracy of quantification [6]. A summary of the research articles of coupling of IPS with various extraction methods for the determination of polar compounds is shown in Table 1.
S.No.
Analyte(s)
Matrix
Derivatizing Reagent
Extraction Technique
Reference
1
Phenols and acidic herbicides
water
MTBSTFA
SBSE
[7]
2
Polyphenols
herbal infusions
BSTFA
DSDME
[8]
3
Melamine and cyanuric acid
powdered milk
BSTFA
LLE
[9]
4
Fluoxetine and norfluoxetine
human plasma
MBTFA
LPME
[10]
5
Fecal sterols
fecal matter
BSTFA
SPE
[11]
6
Quinine
urine
BSTFA+TMCS (99:1v/v)
DLLME
[15]
7
Endocrine disruptor chemicals
wastewater
BSTFA+TMCS (99:1v/v)
DLLME
[16]
8
3-phenoxybenzoic acid
liver and blood
BSTFA+TMCS (99:1v/v)
MISPE-DLLME
[17]
9
Alkylphenols
environmental water samples
BSTFA
MASE and SBSE
[18]
10
Alkylphenols and bisphenol A
seawater samples
BSTFA
SPME
[19]
11
Polycyclic aromatic hydrocarbons
sediment samples
MTBSTFA
SWE and DLLME
[20]
12
Endocrine disrupting chemicals
water
BSTFA+1%TMCS
MEPS
[21]
13
Mono and dicarboxylic acids
ozonolysis of cyclic alkenes
BSTFA
LLE
[22]
14
Chlorinated bisphenol A
human plasma
BSTFA
SPME
[23]
15
Benzophenone UV filters
water
BSTFA
vortex assisted DLLME
[24]
16
Triclosan
wastewater and surface water
TBDMS
SPE
[25]
17
Non-steroidal anti-inflammatory drugs
water samples
TBAHS
ion-pair liquid-liquid extraction
[3]
18
Acidic herbicides
aqueous samples
TBAC
ion-pair hollow fiber-protected LPME
[4]
19
Linear and branched perfluorooctane sulfonate isomers
biological samples
TBAH
SPE
[5]
20
Pharmaceutical residues
water
TBAHS
SPE
[26]
21
Phenolic acids
plasma
TBAH
ion-pair microextraction
[27]
22
Chlorophenoxyacetic acids
water
TBAC
USEME
[28]
23
Linear alkylbenzenesulfonates
aqueous samples
TBAHS
ion-pair-SPME
[29]
24
Low molecular weight dicarboxylic acids
atmospheric aerosols
TBAH
SPE
[30]
25
Long chain fatty acids
water
TBAHS
ion-pair dynami LPME
[31]
SBSE: Stir Bar Sorptive Extraction; DSDME: Directly Suspended Droplet Microextraction; LLE: Liquid-Liquid Extraction; MBTFA: n-methyl-bis(trifluoroacetamide); LPME: Liquid-Phase Microextraction; SPE: Solid Phase Extraction; MASE: Membrane Assisted Solvent Extraction; SWE: Subcritical Water Extraction; MEPS: Microextraction by Packed Sorbents; TBDMS: tert-butyldimethylsilylated; USEME: Ultrasound Assisted Emulsification Microextraction.
Table 1: Coupling of IPD with various extraction methods in literature.
Applications of IPS
The derivatization using IPS for GC analysis of 46 acidic and polar pollutants including phenols, acidic herbicides and several pharmaceuticals extracted from water samples [7]. Three derivatization strategies such as silylation, acetylation and alkylation tested for the analysis of all the targeted analytes. N-(tert-butyldimethylsilyl)-N-methyltrifluoroacetamide, MTBSTFA (silylating reagent) was found to give best results for the simultaneous analysis of 46 acidic and polar pollutants using IPS. The high pH need for in-situ acetylation decreased the extraction efficiency of pharmaceutical herbicides, because phenols could not derivatize with alkylating reagent such as tetrabutylammonium salt [7].
Several factors such as mode of injection, injector port temperature and derivatization time influence the yield of IPS as studied by several authors [8-11]. In one such study, Vinas and the co-workers [8] have used BSTFA for IPS of polyphenols and compared the mode of injection either split and split-less and later was found superior over former. The temperature of GC-MS injector port between 160–280°C was also screened. The yield of derivatization of all polyphenols was found to increase upto 240°C and this temperature was found most suitable for the IPS derivatization of polyphenols [8]. The injector port temperature has played a critical role during IPS. Tzing and Ding [9] have shown that as the temperature raises from 75 to 90°C, the derivatization yield increased; which tends to decrease further after 90°C for the analytes melamine and cyanuric acid with BSTFA containing 1%TMCS. The residence time, i.e. time required for the analytes to react with derivatizing reagent inside the GC-MS injection port was also evaluated and found that 2 min giving the optimum derivatization efficiency. In another study conducted to evaluate the effect of solvents used for IPS for fluoxetine and norfluoxetine have shown that less volatile solvents were able to give satisfactory repeatability of the derivatization. Apart from these, initial column temperature and carrier gas flow rate has shown to effect the yield of derivatization using N-methyl- bis(trifluoroacetamide), MBTFA as injector-port derivatizing agent [10]. In another study, Wu et al [11], has also investigated certain parameters like effect of solvents such as acetonitrile, acetone, dichloromethane, diethyl ether, ethyl acetate, hexane and tert-butyl methyl ether and shown that dichloromethane giving the best derivatization efficiency after solid-phase extraction of fecal sterols from environmental water samples by IPS/GC-MS analysis. Based on the literature and usefulness of the IPS as an easy to use derivatization method, it has expanded its scope for analysis of polar analytes using GC-MS.
DLLME-IPS
In recent years, microextraction techniques coupled with different derivatization make the analysis more efficient, sensitive, selective, economical and eco-friendly. Dispersive liquid-liquid microextraction (DLLME), a new microextraction technique introduced by Assadi and co workers [12] has gained a promising place among the researchers to develop rapid and cost-effective sample preparation methods for the analytes of their interest and improve this technique thereupon. This method mainly based on ternary component solvent system in which an appropriate mixture of dispersant, extraction solvent (both miscible in each other) rapidly injected into an aqueous solution which enable the formation of a cloudy solution (water/ dispersant/extraction solvent). This cloudy solution has tiny droplets of extraction solvent dispersed throughout the aqueous solution. The hydrophobic analytes are then enriched in the extraction solvent is centrifuged, due to which, high density extraction solvent accumulates at the bottom of the tube known as sedimented phase which can directly injected into GC for analysis. Compared with SPME and SDME, the extraction time in DLLME is very less. DLLME has been widely applied for the analysis of organic analytes and metals from various complex matrices [13, 14].
An attempt has made by our group to couple DLLME with injector port silylation (IPS) which can enhance the scope of DLLME for the analysis of polar analytes at cheaper cost. This coupling enhances the use of DLLME and overcome several limitations of in-vial silylation. This coupling lessens the (a) time for silylation (less than a minute), (b) need of external anhydrous conditions, (c) use of toxic silylating reagent and the solvents used for extraction. The coupling of DLLME with IPS has successfully applied for extraction of quinine from urine samples and the sediment phase then injected manually into GC-MS along with BSTFA containing 1% TMCS. Thus, quinine was derivatized inside the hot GC-MS injector port instantaneously thus eliminating the lengthy reaction time needed in conventional in-vial silylation [15]. The DLLMEIPS also used for the analysis of multi-class analytes like phenolic endocrine disruptors (PEDCs) in environmental water samples. This method added the advantage of automatic injection of both sample and derivatizing agent using an auto sampler which eliminates the need of injecting them manually into the GC [16]. In another study the DLLME-IPS has hyphenated with molecularly imprinted polymers (MIP) (has ability for selective picking of the analytes from the sample) for the quantitative determination of 3-phenoxybenzoic acid (3-PBA) from complex biological samples such as blood and liver. This has improved not only sensitivity but also enhanced the selectivity of the analysis. The analyte, 3-PBA has been extracted from biological samples using molecularly imprinted polymer (MIP) solid-phase extraction (MISPE) [17]. The DLLME-IPS-GC-MS approach has been shown in Figure 1.
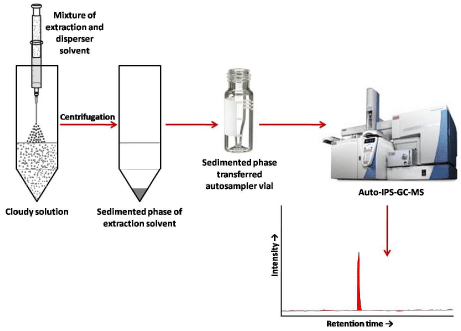
Figure 1: Diagrammatic representation of DLLME-IPS-GC-MS approach.
Conclusion and Future Directions
Coupling of DLLME with IPS results in a rapid, economical, eco-friendly and sensitive analytical method. This coupling has enabled to analyze polar analytes by GC-MS. It is a first step in coupling the microextractions with injector port derivatization but need more such. DLLME-IPS has the potential to analyze multiple polar analytes in single run due to the potential of DLLME as extraction/preconcentration tool and ability of silylation as an effective derivatization agent for most of the polar analytes which definitely expand the use of GC-MS for toxicological and/or clinical analysis. In future, DLLME-IPS/GC-MS could be an alternative to study the polar analytes in simple or complex matrices for several studies including untargeted metabolomics. The researchers should concentrate in this area of research so that the methods developed will be helpful for the routine analysis and generate more authentic data for regulatory purposes. These methods can also cut the burden on the analyst who is performing day-to-day analysis in the laboratory. Further, this approach reduces the use of toxic organic solvents for extraction and thus develops eco-friendly method, a step towards green chemistry.
Acknowledgements
The authors are thankful to Dr CS Nautiyal, the Director, CSIRIITR, Lucknow for his support. Authors are acknowledging the financial support provided by DFAT through ‘Safe Water” PSLP project (WBS-R-03241-01) between CSIRO Land & Water Flagship, Australia and CSIR-IITR, Lucknow, India. RJ is thankful to Dr. S. K. Jain, Dy. Director-cum-Coordinator, CFSL, Guwahati for his constant support.
References
- Schummer C, Delhomme O, Appenzeller BM, Wennig R, Millet M. Comparison of MTBSTFA and BSTFA in derivatization reactions of polar compounds prior to GC/MS analysis. Talanta. 2009; 77: 1473-1482.
- Rasmussen KE. Quantitative morphine assay by means of gas-liquid chromatography and on-column silylation. J Chromatogr. 1976; 120: 491-495.
- Xu L, Jiang M, Li G. Injection port derivatization following sonication-assisted ion-pair liquid-liquid extraction of nonsteroidal anti-inflammatory drugs. Anal Chim Acta. 2010; 666: 45-50.
- Wu J, Lee HK. Injection port derivatization following ion-pair hollow fiber-protected liquid-phase microextraction for determining acidic herbicides by gas chromatography/mass spectrometry. Anal Chem. 2006; 78: 7292-7301.
- Chu S, Letcher RJ. Linear and branched perfluorooctano sulfonate isomers in technical product and environmental samples by in-port derivatization-gas chromatography-mass spectrometry. Anal. Chem. 2009; 81: 4256-4262.
- Wang Q, Ma L, Yin CR, Xu L. Developments in injection port derivatization. J Chromatogr A. 2013; 1296: 25-35.
- Quintana JB, Rodil R, Muniategui-Lorenzo S, López-Mahía P, Prada-Rodríguez D. Multiresidue analysis of acidic and polar organic contaminants in water samples by stir-bar sorptive extraction-liquid desorption-gas chromatography-mass spectrometry. J Chromatogr A. 2007; 1174: 27-39.
- Vinas P, Castillo NM, Campillo N, Cordoba MH. Directly suspended droplet microextraction with in injection-port derivatization coupled to gas chromatography-mass spectrometry for the analysis of polyphenols in herbal infusions, fruits and functional foods. J. Chromatogr. A 2011; 1218: 639-646.
- Tzing SH, Ding WH. Determination of melamine and cyanuric acid in powdered milk using injection-port derivatization and gas chromatography-tandem mass spectrometry with furan chemical ionization. J. Chromatogr. A 2010; 1217: 6267-6273.
- Oliveira AF, de Figueiredo EC, Dos Santos-Neto AJ. Analysis of fluoxetine and norfluoxetine in human plasma by liquid-phase microextraction and injection port derivatization GC-MS. J Pharm Biomed Anal. 2013; 73: 53-58.
- Wu J, Hu R, Yue J, Yang Z, Zhang L. Determination of fecal sterols by gas chromatography-mass spectrometry with solid-phase extraction and injection-port derivatization. J Chromatogr A. 2009; 1216: 1053-1058.
- Rezaee M, Assadi Y, Milani Hosseini MR, Aghaee E, Ahmadi F, Berijani S. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J Chromatogr A. 2006; 1116: 1-9.
- Herrera-Herrera AV, Ramos MA, Borges JH, Delgado MAR. Dispersive liquid-liquid microextraction for determination of organic analytes. Trends Anal. Chem. 2010; 29: 728-751.
- Shahawi MSE, Al-Saidi HM. Dispersive liquid-liquid microextraction for chemical speciation and determination of ultra-trace concentration of metal ions. Trends Anal. Chem. 2013; 44: 12-24.
- Jain R, Mudiam MKR, Ch R, Chauhan A, Khan HA, Murthy RC. Ultrasound-assisted dispersive liquid-liquid microextraction followed by injector port silylation: A novel method for rapid determination of quinine in urine by gas chromatography-mass spectrometry. Bioanalysis. 2013; 5: 2277-2286.
- Mudiam MKR, Jain R, Singh R. Application of ultrasound assisted-dispersive liquid-liquid microextraction and automated in-port silylation for the simultaneous determination of phenolic endocrine disruptor chemicals in water samples by gas chromatography-triple quadrupole mass spectrometry. Anal. Meth. 2014; 6: 1802-1810.
- Mudiam MKR, Chauhan AK, Jain R, Dhuriya YK, Saxena PN, Khanna VK. Molecularly imprinted polymer coupled with dispersive liquid-liquid microextraction and injector port silylation: A novel approach for the determination of 3-phenoxybenzoic acid in complex biological samples using gas chromatography-mass spectrometry. J. Chromatogr. B 2014; 945-946: 23-30.
- Cavalheiro J, Monperrus M, Amouroux D, Preud'Homme H, Prieto A, Zuloaga O. In-port derivatization coupled to different extraction techniques for the determination of alkylphenols in environmental water samples. J Chromatogr A. 2014; 1340: 1-7.
- Basheer C, Parthiban A, Jayaraman A, Lee HK, Valiyaveettil S. Determination of alkylphenols and bisphenol-A a comparative investigation of functional polymer-coated membrane microextraction and solid-phase microextraction techniques. J Chromatogr. A 2005; 1087: 274-282.
- Wang X, Lin L, Luan T, Yang L, Tam NFY. Determination of hydroxylated metabolites of polycyclic aromatic hydrocarbons in sediment samples by combining subcritical water extraction and dispersive liquid-liquid microextraction with derivatization. Anal. Chim. Acta 2012; 753: 57-63.
- Prieto A, Vallejo A, Zuloaga O, Paschke A, Sellergen B, Schillinger E, Schrader S, et al. Selective determination of estrogenic compounds in water by microextraction by packed sorbents and a molecularly imprinted polymer coupled with large volume injection-in-port-derivatization gas chromatography-mass spectrometry. Anal. Chim. Acta. 2011; 703: 41-51.
- Docherty KS, Zieman PJ. On-line, inlet-based trimethylsilyl derivatization for gas chromatography of mono- and dicarboxylic acids. J Chromatogr A. 2001; 921: 265-275.
- Olmo MD, Zafra A, Suarez B, Casado AG, Taoufiki J, Vilchez J.L. Use of solid-phase microextraction followed by on-column silylation for determination chlorinated bisphenol A in human plasma by gas chromatography-mass spectrometry. J. Chromatogr. B 2005; 817: 167-172.
- Zhang Y, Lee HK. Determination of ultraviolet filters in water samples by vortex-assisted dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry. J Chromatogr A. 2012; 1249: 25-31.
- Cheng CY, Wang YC, Ding WH. Determination of triclosan in aqueous samples using solid-phase extraction followed by on-line derivatization gas chromatography-mass spectrometry. Anal Sci. 2011; 27: 197-202.
- Lin WC, Chen HC, Ding WH. Determination of pharmaceutical residues in waters by solid-phase extraction and large-volume on-line derivatization with gas chromatography-mass spectrometry. J Chromatogr A. 2005; 1065: 279-285.
- Peters S, Kaal E, Horsting I, Janssen HG. An automated method for the analysis of phenolic acids in plasma based on ion-pairing micro-extraction coupled on-line to gas chromatography/mass spectrometry with in-liner derivatization. J. Chromatogr. A 2012; 1276: 71-76.
- Y Yamini, Saleh A. Ultrasound-assisted emulsification microextraction combined with injection-port derivatization for the determination of some chlorophenoxyacetic acids in water samples. J. Sep. Sci. 2013; 36: 2330-2338.
- Alzaga R, Peña A, Ortiz L, Bayona JM. Determination of linear alkylbenzensulfonates in aqueous matrices by ion-pair solid-phase microextraction-in-port derivatization-gas chromatography-mass spectrometry. J Chromatogr A. 2003; 999: 51-60.
- Hsu CL, Ding WH. Determination of low-molecular-weight dicarboxylic acids in atmospheric aerosols by injection-port derivatization and gas chromatography-mass spectrometry. Talanta. 2009; 80: 1025-1028.
- Wu J, Lee HK. Ion-pair dynamic liquid-phase microextraction combined with injection-port derivatization for the determination of long-chain fatty acids in water samples. J Chromatogr A. 2006; 1133: 13-20.