
Rapid Communication
Austin J Anal Pharm Chem. 2018; 5(3): 1107.
Synthesis of a New Chiral Organocatalysts and its Application in Asymmetric Morita-Baylis-Hillman Reaction
Yu Qiuhan, Lu Weiwen, Ding Zhiqiang, Wei Min and Dai Zhenya*
Department of Medicinal Chemistry, School of Pharmacy, China Pharmaceutical University, 210009 Nanjing, P. R. China
*Corresponding author: Dai Zhenya, Department of Medicinal Chemistry, School of Pharmacy, China Pharmaceutical University, 210009 Nanjing, P. R. China
Received: September 18, 2018; Accepted: October 09, 2018; Published: October 16, 2018
Introduction
The Morita–Baylis–Hillman (MBH) reaction was an organocatalyzed chemical transformation that provided effective and atom economical carbon–carbon bond-forming reactions [1-5]. Formally, this reaction promoted condensation between α-position of an electron-deficient alkene and the sp2 carbon atom of an aldehyde catalyzed by nucleophilic bases such as DABCO [5]. With highly functionalized MBH adducts and their derivatives, structurally complex and diverse molecules (such as acaterin, asmarines A and B, borrelidin, PPAPs and so many natural products [4,6]) could be easily achieved. Hence, the development of the MBH reaction has attracted considerable interest in recent years. However, several disadvantages such as poor conversions, low reaction rates, low enantioselectivity and the lack of definite mechanism also limited the applicability of the MBH reactions [2,4,6,7]. Therefore, it is important to investigate new catalytic system to solve known problems.
Results and Discussion
For the asymmetric MBH reaction, thiourea derivatives were widely used {7-12]. Besides, cyclohexanediamine and proline were both important chiral frameworks. Therefore, we combined these two structural units and synthesized a new catalyst C (Figure 1, Table 1).
In comparison to DABCO, there was no product when the reaction was carried out with DMAP as base. Meanwhile, the yield with DBU as base was much lower though DBU played an important role in the MBH reaction [13].
For further optimization, the effect of the solvents on the reaction was also investigated (Table 2).
Obviously, for this reaction, the presence of solvent exerted a tremendous influence.
Therefore, in order to get superior yields, DABCO should be used as the base while DCM should be chosen as the solvent.
According to the best condition we got above, many other reactions had been tested. Besides, optical rotation also had tested, and from Table 3, we could see that the selectivity of the catalyst was not satisfying. Thus further work should be done to improve the enantioselectivity of the reaction.
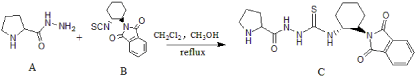
Figure 1: Synthesis of catalyst.
Table 1: Optimization of the Base in the Reaction of 2-Cyclohexen-1-one with benzaldehyde.
Table 2: Solvent Effects on the MBH Reaction of 2-Cyclohexen-1-one with benzaldehyde.
Table 3: MBH Reaction of Enones and Acrylates with Aldehydes Catalyzed by C.
Experimental
Materials and measurements
All reagents and solvents were chemically pure (CP) grade or analytical reagent (AR) and were used as received unless otherwise indicated general. 1H NMR and 13C NMR spectra were measured on a Bruker AV 500 spectrometer at 303k from sample solution in CDCl3. Mass spectra were measured on a Waters Q-TOF micro spectrometer.
Synthesis of C [14-16]
In a flask, A (0.1508g) and B (0.076g) was dissolved in DCM (10mL) and CH3OH (1ml). The mixture was refluxed overnight. After concentrated, the residue was subjected to flash column chromatography (silica gel thin layer chromatography; mineral ether: ethyl acetate 2:1) to give dark yellow powder. Yield: 0.1463g (25.67%).
1H NMR (300 MHz, CDCl3) δ 7.77 (s, 2H), 7.65 (s, 2H), 4.04 (s, 1H), 3.40 (s, 1H), 3.26 (s, 1H), 2.60 (dd, 2H), 1.99 (d, 3H), 1.93 (s, 2H), 1.84 (s, 2H), 1.79 (s, 2H), 1.49 (dd, 2H), 1.39 – 1.12 (m, 4H).
13C NMR (75 MHz, CDCl3) δ 182.07 (s), 136.18 (s), 134.71 (s), 126.19 (s), 79.62 (s), 65.75 (s), 58.70 (s), 58.34 (s), 57.72 (d, J = 15.3 Hz), 54.28 (s), 35.89 (s), 31.89 (s), 30.96 (s), 28.38 (s), 27.12 (s), 26.68 (s).
General procedure for the synthesis of J1-3 [12]
The reaction was carried out with 1 equiv of aldehyde and 4 equiv of enone or acrylate in the presence of 20mol% catalyst and base at 10oC for 72h. After concentrated, the residue was subjected to flash column chromatography to get product.
References
- Zhao MX, WY, Shi M. Chapter 2: Catalytic Systems for the Morita–Baylis–Hillman Reaction. Rsc Catalysis. 2011; 79-208.
- Sasai H, TS. 6.9 C–C Bond Formation: (aza) Morita–Baylis–Hillman Reaction [Comprehensive Chirality]. 2012; 6: 234-263.
- Coelho MS. Mechanistic Options for the Morita–Baylis–Hillman Reaction (n → π*. Lewis Base Catalysis in Organic Synthesis, ed. E.V.S.E. Denmark. 2016; 191-232.
- Basavaiah D, Reddy BS & Badsara SS. Recent Contributions from the Baylis−Hillman Reaction to Organic Chemistry. Chemical Reviews. 2010; 110: p. 5447–5674.
- Ciganek E. The Catalyzed α‐Hydroxyalkylation and α‐Aminoalkylation of Activated Olefins (The Morita—Baylis—Hillman Reaction) Organic Reactions. 1997: John Wiley and Sons, Inc.
- Wang F-j, Y Wei and M Shi. Application of Morita–Baylis–Hillman Reaction for the Synthesis of Natural Products. 2011: 485-551.
- Sohtome Y, et al. Development of bis-thiourea-type organocatalyst for asymmetric Baylis–Hillman reaction. Tetrahedron Letters. 2004; 45: p. 5589-5592.
- Zhang H, et al. 4-Aminothiourea Prolinoltert-Butyldiphenylsilyl Ether: A Chiral Secondary Amine-Thiourea as Organocatalyst for Enantioselectiveanti-Mannich Reactions. Advanced Synthesis & Catalysis. 2009; 351: p. 2288-2294.
- Lalonde MP, Y Chen and EN Jacobsen. A Chiral Primary Amine Thiourea Catalyst for the Highly Enantioselective Direct Conjugate Addition of α,α-Disubstituted Aldehydes to Nitroalkenes. Angewandte Chemie, 2006; 118: p. 6514-6518.
- Farley AJ, C Sandford and DJ Dixon. Bifunctional Iminophosphorane Catalyzed Enantioselective Sulfa-Michael Addition to Unactivated alpha-Substituted Acrylate Esters. J Am Chem Soc. 2015; 137: p. 15992-15995.
- Chen Q, et al. An asymmetric approach toward chiral multicyclic spirooxindoles from isothiocyanato oxindoles and unsaturated pyrazolones by a chiral tertiary amine thiourea catalyst. Chem Commun (Camb). 2013; 49: p. 1657-1659.
- Albrecht Berkessel KR and Jo1rg M Neudo1rfl. Asymmetric Morita-Baylis-Hillman Reaction Catalyzed by Isophoronediamine-Derived Bis(thio)urea Organocatalysts. ORGANIC LETTERS. 2006; 8: p. 4195-4198.
- Aggarwal VK & Mereu. Superior amine catalysts for the Baylis–Hillman reaction_ the use of DBU and its implications†. Chemical Communications. 1999; 22: p. 2311–2312.
- Bhowmick S, SS Kunte and KC Bhowmick. The smallest organocatalyst in highly enantioselective direct aldol reaction in wet solvent-free conditions. RSC Adv. 2014; 4: p. 24311-24315.
- Mlostoń G, et al. Studies on the synthesis and some reactions of (S)-proline hydrazides. Tetrahedron: Asymmetry. 2012; 23: p. 795-801.
- Wei S, et al. New highly enantioselective thiourea-based bifunctional organocatalysts for nitro-Michael addition reactions. Catalysis Today. 2007; 121: p. 151-157.