
Review Article
Austin Biomark Diagn. 2015;2(1): 1016.
Adipokines in Hepatic Angiogenesis
Michal Kukla*, Marek Waluga and Edward Surma
Department of Gastroenterology and Hepatology, Medical University of Silesia, Poland
*Corresponding author: Michal Kukla, Department of Gastroenterology and Hepatology, Medical University of Silesia, ul. Medykow 14, 40-752 Katowice, Poland,
Received: December 15, 2014; Accepted: March 16, 2015; Published: March 24, 2015
Abstract
Hepatic angiogenesis in the course of chronic hepatitis merely represents a homeostatic mechanism directed to ensuring a necessary oxygen supply or one that plays an additional pathogenic role contributing to liver damage. Additionally, in chronic hepatitis, there is a switch toward proangiogenic factors. Many efforts have been directed to explain the mechanisms involved in angiogenesis during the progression of liver fibrosis. Recent data indicate that hepatic angiogenesis and fibrosis are closely related in both clinical and experimental conditions. Adipokines not only regulate adipose tissue and glucose metabolism, but also influence inflammation, fibrogenesis and production of proangiogenic agents. This short review briefly described a possible role of some adipokines in hepatic neovessels formation during liver morbidity.
Keywords: Adipokines; Angiogenesis; Chronic hepatitis; Liver; Fibrosis; Visfatin
Introduction
Angiogenesis, the formation of new vascular structures from preexisting vessels, occurs in Chronic Liver Diseases (CLDs) [1,2]. Hepatic angiogenesis in the course of chronic hepatitis merely represents a homeostatic mechanism directed to ensuring a necessary oxygen supply or one that plays an additional pathogenic role contributing to liver damage [3,4].
Angiogenesis in CLDs can result from two pathogenic pathways (Figure 1). Firstly, neo-angiogenesis is evoked and potentiated in hepatic tissue by progressive tissue hypoxia. Accumulation of inflammatory cells and development of fibrosis may enhance resistance of liver tissue to blood flow and oxygen supply, resulting into hypoxia [5]. On the other hand accumulated evidences indicate hypoxia alone could be important in the stimulation of angiogenesis and can also stimulate inflammation leading to a viscous circle between inflammation and angiogenesis [6-8]. Hypoxia activates angiogenesis as a result of signaling mediated by Hypoxia-Inducible Factors (HIFs) [7-10]. Also hepatic steatosis enhances disturbances in hepatocyte energy regulation provoking hypoxia and cell injury with subsequent development of neoangiogenesis [11,12]. These circumstances contribute to an up-regulation of proangiogenic factors leading to vascular remodeling and neovessels formation [13]. Secondly, the process of liver chronic wound healing is a hallmark for fibrogenic CLDs. It is associated with an increased expression of growth factors, cytokines and Metalloproteinases (MMPs) with an underlying proangiogenic activity [14]. Angiogenesis in liver is characterized by capillarization of the sinusoids [15]. Structures responsible for proliferation and maturation of new blood vessels in the liver are Hepatic Stellate Cells (HSCs), Kupffer cells, regenerating hepatocytes and existing Endothelial Cells (ECs) [3].
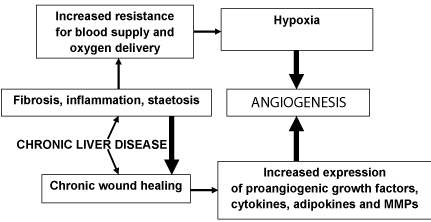
Figure 1: Two main pathways of angiogenesis in fibrogenic chronic liver
diseases; MMPs – matrix metaloproteinases.
Angiogenic process in the liver may be peculiar due to: 1) the existence of two different types of microvascular structures in the liver – large vessels covered by a continuous endothelium and sinusoids lined by a fenestrated endothelium [16] and 2) the production of unique proangiogenic factors, such as Angiopoietin- Like 3 (ANGPTL3), a liver specific secreted factor showing angiogenic properties by binding to avβ3 integrin [17]; and 3) the presence of phenotypically and functionally specific HSCs-considered liver – resident pericytes, which may stimulate angiogenesis through mechanisms different from those attributed to microcapillary pericytes [18]. Additionally new factors involved in pathological neovascularization in cirrhosis like up-regulation of Aquaporins (AQPs) - an integral membrane water channels which enhances osmotic water permeability and Fibroblast Growth Factor (FGF)- induced dynamic membrane blabbing in liver endothelial cells are emerging on horizon [19]. However a key area in the study of cellular and molecular relationship existing between fibrogenesis and angiogenesis concerns the proangiogenic role of activated HSCs, like some of the adipocytokines (adipokines) [18].
Adipokines, adipose tissue derived hormones, have been shown not only to be regulators of fibrogenesis, metabolic and inflammatory processes but also as potent regulators of angiogenesis. They may influence structures and regulate synthesis of agents responsible for modulation of angiogenesis (Table 1) [20,21]. Leptin, visfatin, chemerin and resistin have been found to promote angiogenesis, whereas adiponectin attenuates it [22-27]. Data regarding vaspin (visceral adipose tissue-derived serine protease inhibitor) and apelin is very scare [28].
Leptin
Resistin
Visfatin
Activation and stimulation of HSCs. Inhibition of HSCs apoptosis
Stimulation of VEGF and VEGFR production
Stimulation of synthesis and activation of MMPs
Stimulation of VEGF and VEGFR production
Stimulation of synthesis and activation of MMPs
Stimulation of VEGF production
Enhancement of ECs proliferation
Increase of adhesion molecules
Inhibition of TIMPs
Generation of ROS and oxidative stress
Activation and stimulation of HSCs
Enhancement of ECs proliferation, migration and blood vessels formation
Activation of mTOR
Enhancement of ECs migration and blood vessels formation
Decrease of ECs apoptosis
Stabilization of HIF 1a
Generation of ROS
Increase of aSMA and procollagen type 1 synthesis. Acceleration of fibrosis
Stimulation of proinflammatory cytokines
Stimulation of proinflammatory cytokines
Activation of PI3K and PKB/Akt
Increase of adhesion molecules synthesis
Table 1: Proangiogenic action of adipokines.
The first discovered and till now the most extensively studied adipokine leptin has been shown to be involved in regulation of fibroproliferative processes and angiogenesis in chronic liver diseases [20,21,29].
Increased levels of circulating leptin have been found in Chronic Hepatitis C (CHC) patients when compared to healthy controls [30- 32]. Uygun et al. reported that plasma concentrations of leptin are increased in Nonalcoholic Steatohepatitis (NASH), independently of Body Mass Index (BMI) [33]. Moreover, in patients with chronic viral liver disease, serum leptin levels tend to increase as liver function worsens [34,35].
Activation of leptin receptors in HSCs leads to increase of Vascular Endothelial Growth Factor (VEGF) expression [22] (Table 2). It also exerts a direct angiogenic action on endothelial cells which express the functional long term form of leptin receptor [23]. After induction of fibrosis in rat the specific leptin receptor ObR was found to be co-localized with receptors for VEGF and a-Smooth Muscle Actin (a-SMA) [30,36]. Pro-angiogenic role of leptin involves both activation of the mammalian Target Of Rapamycin (mTOR) pathway and generation of Reactive Oxygen Species (ROS) via Nicotinamide Adenine Dinucleotide Phosphate (NADPH)-oxidase, the latter being relevant for HIF-1a stabilization but not for mTOR activation [30,36,37].
Inhibition of HSCs proliferation and migration
Decrease of aSMA and procollagen type 1 synthesis. Down-regulation of fibrosis
Reduction of ROS synthesis and oxidative stress
Inhibition of proinflammatory cytokines
Activation of AMPK
Reduction of PDGF
Inhibition of ECs proliferation, migration and survival. Reduction of vessels stabilization and pericytes recruitment.
Table 2: Antiangiogenic role of Adiponectin.
Leptin promotes endothelial cell tube formation and up-regulates VEGF mRNA expression via activation of the Jak/Stat3 signaling pathway [38]. Stimulation of neovessels formation in the liver by leptin is consistent with its profibrogenic role, as angiogenesis is considered a relevant component of the progression of liver damage and has been described in the context of inflammation and fibrogenesis in CLDs [3,21]. Leptin also modulates VEGF-induced vascular activity by synergistically promoting neovascularization in vivo [39]. These
observations suggest that leptin acts not only as a direct but also as an indirect angiogenic factor or modulator of other angiogenic agents. Leptin mRNA expression in adipocytes was markedly increased by hypoxia. Activation of leptin gene expression by hypoxia clearly resulted in an increase of leptin release. Up-regulated leptin production under hypoxic conditions would reflect an adaptive mechanism that promotes angiogenesis [40]. Serum levels of leptin in CHC were significantly increased compared to healthy controls and presented positive correlation with grade of inflammatory activity [41]. Other authors reported its positive association with fibrosis stage [42] and higher levels in the case of cirrhosis [43]. However, there was no association between serum leptin and angiogenesis intensity either in portal tracts or hepatic lobules in CHC patients. Nevertheless, leptin was significantly higher in CHC patients with minimal inflammatory activity and was inversely correlated with inflammatory grade. So, the lack of the association between leptin serum concentrations and hepatic angiogenesis in CHC may result from the direct influence of viral infection and ongoing inflammatory process on serum levels of this adipokine. Stimulation of neovessel formation in the liver by leptin is consistent with its profibrogenic role, as angiogenesis is a relevant component of chronic wound healing [44]. On the other hand, the well-established association between angiogenesis and tumorigenesis suggests a possible additional role of leptin in liver cancer. Along these lines, recent data implicate leptin in the progression of Hepatocellular Carcinoma (HCC), since as leptin increases growth, migration and invasiveness of HCC cell lines [45]. Additionally, leptin stimulates proliferation and metastatic potential of cholangiocarcinoma cells [46,47].
Adiponectin generally exerts hepatoprotective effect in CLDs and protects against inflammation and fibrosis progression. Numerous studies showed lower serum adiponectin concentrations in NAFLD patients when compared to matched controls [48-52]. Intrahepatic expression of mRNA for adiponectin and its receptors AdipoR2 in NAFLD patients were found to be lower in NASH patients compared to subjects with simple steatosis [48,49]. Also in CHC reduction of serum adiponectin concentration has been reported [53]. Protective role of adiponectin results from inhibition of pro-inflammatory and induction of anti-inflammatory cytokines in leukocytes and ECs. The correlation between plasma adiponectin levels and the severity of liver damage is still controversial. Moreover, there are some data indicating that serum adiponectin correlates with HCV viral load and genotype but not with histological abnormalities in patients with CHC [54]. Adiponectin has been found to activate AMPActivated Protein Kinase (AMPK) in HSCs. AMPK activation leads to inhibition of Platelet-Derived Growth Factor (PDGF) expression, which is essential in ECs proliferation, nascent vessel stabilization and pericytes recruitment [55]. Moreover, adiponectin inhibits ECs migration and survival via activation of apoptosis [27]. Adiponectin has been found to markedly diminish HSCs proliferation and a-SMA and protects against fibrosis [56]. There was no association between serum adiponectin and hepatic angiogenesis intensity in CHC patients. However adiponectin was inversely related to inflammatory activity. The ongoing inflammatory process which modulates serum adiponectin levels may interfere with the relationship between adiponectin and angiogenesis.
A novel adipokine visfatin/Pre-B cell colony-Enhancing Factor (PBEF)-1 has been shown to be altered in CLDs [34,57] and associated with liver fibrosis [58-60]. The ability of visfatin to induce expression of genes and proteins for Matrix Metalloproteinases (MMP-2 and MMP-9), VEGF and its Receptor (VEGF-R2) in Human Umbilical Vein Endothelial Cells (HUVECs) in a dose dependent manner acknowledges its role in pathogenesis of chronic hepatitis and angiogenesis. Simultaneously, visfatin inhibits expression of genes and proteins for Tissue Inhibitors of Matrix Metallproteinases (TIMP) - TIMP-1 and TIMP-2. Inhibition of VEGF and VEFG-R2 results in down-regulation of MMPs expression induced by visfatin [61]. Visfatin potentiates proliferation, migration of endothelial cells and formation of new blood vessels in a dose dependent manner. Moreover, it decreases apoptosis of ECs. Visfatin influences angiogenic process by activation of Phosphatidylinositol 3-Kinase (PI3K), protein kinase B (PKB/Akt) i ERK1/2 (extracellular signalregulated kinase 1/2, p42/p44 Mitogen-Activated Protein Kinase, p42/ p44 MAPK) [61]. Only one study of CHC patients has found serum visfatin to be significantly increased in CHC and negatively associated with inflammatory activity [57]. There was no association between serum visfatin levels and angiogenesis intensity, assessed by CD34 hepatic expression, expression in a whole group of CHC patients [62]. Surprisingly, visfatin was inversely related to angiogenesis intensity in hepatic lobules and portal tracts in females. This observation is even more interesting because there was no difference in serum visfatin concentration between men and women. The explanation of these results is difficult and requires further investigations. However, they appear to indicate that the role of adipokine in angiogenesis may differ in men and women [62].
Serum chemerin concentrations were higher in CHC patients compared to the control group and inversely associated with inflammatory activity [63]. There was no difference in CHC patients with different fibrosis stage and steatosis grade. Serum chemerin concentration was significantly higher in NAFLD patients compared to healthy volunteers. Serum chemerin was significantly higher in patients with NASH compared to patients with simple steatosis and positively related to inflammatory activity [64].
Recently chemerin has been shown to promote angiogenesis in endothelial cells in a dose dependent manner [65]. Chemerin activates the pathway dependent on PI3K/Akt and MAPK in ECs, activating angiogenesis and MMPs synthesis [25]. However, serum chemerin concentration in CHC patients did not show any association with hepatic angiogenesis intensity (CD34 expression) [62].
Resistin enhances in vitro human endothelial cell proliferation and migration. Moreover, resistin evokes capillary-like tube formation, increases mRNA expression of some angiogenesis-related factors such as VEGF [66], Vascular Endothelial Growth Factor Receptors (VEGFR-1 and VEGFR-2), MMP-1 and MMP-2, Vascular Cell Adhesion Molecule-1 (VCAM-1) and endothelin-1 [67], and activates ERK1/2 and p38 pathways [68]. Migration of murine endothelial cells migration and sprouting of cellular networks seems to be regulated by resistin via a mechanism which appears too dependent on PI3K and nuclear factor (NF)-κB activity, but independent of NO production [28]. All above mentioned processes point to a pivotal role of resistin angiogenesis regulation. Additionally, resistin stimulates HSCs to production chemokines with proinflammatory action such as Monocyte Chemotactic Protein-1 (MCP-1) and IL-8, which are suggested to be critical mediators of intrahepatic leukocyte recruitment [69]. The results regarding resistin in chronic hepatitis are ambiguous. The study by Tsochatzis et al. showed higher serum resistin levels in patients with chronic hepatitis B and C than with NASH [70]. Bertolani et al. revealed resistin intrahepatic mRNA levels significantly higher in patients with alcoholic hepatitis compared to the control group, but not to those with CHC or NASH [69]. However, there was no association between serum resistin and the number of new blood vessels in the liver of CHC patients [62].
Serum vaspin was lower in CHC patients without or with not advanced fibrosis. It was up-regulated in patients with advanced fibrosis. Antiviral therapy did not alter vaspin serum concentration regardless of its efficacy [71]. The studies on NAFLD showed upregulated serum vaspin levels regardless of potential confounders [64,72]. Serum vaspin concentration was significantly up-regulated in NASH as compared to pure steatosis [64].
Administration of vaspin to obese ICR mice fed with high fat and sucrose chow inhibited TNF-a, leptin and resistin expression in mesenteric and subcutaneus white adipose tissues [73].
Although vaspin is mainly confined to the adipocytes, it may influence endothelial cells in a similar manner to another proteinase inhibitor - Plasminogen Activator Inhibitor-1 (PAI-1), which is derived from mesenteric fat and suppresses endothelial cell migration and angiogenic branching [44,45]. However, vaspin had no effects on both basal ECs morphology and their TNF-a-induced morphological damage [44,45]. Furthermore, vaspin did not decrease the TNF-a induction of VCAM-1, intercellular adhesion molecule-1 and endothelial selectin expression. Fu and al.’s study indicates that vaspin has no effects on normal ECs, and cannot prevent TNF-a- induced inflammatory injury [28]. Contrary, the study by Jung et al. revealed that vaspin might attenuate the cytokine-induced expression of adhesion molecule genes by inhibiting NF-κB following AMPK activation in Ecs [74]. Additionally vaspin inhibits ECs apoptosis by preventing caspase-3 activation and the inhibition of ROS generation [75]. The study by Liu et al. showed that vaspin inhibited TNF-a and interleukin (IL)-1 mediated activation of NF-κB and its downstream molecules in a concentration-dependent manner in ECs. Therefore vaspin protected endothelial cells from proinflammatory cytokines induced inflammation [76]. Serum vaspin levels were significantly and positively associated with angiogenesis intensity both in lobules and portal tracts in CHC patients [62].
Further studies are needed to elucidate the specific mechanism by which vaspin may influence angiogenic process keeping in mind its anti-inflammatory and antiatherogenic activity from one side and protective role for ECs on the other hand.
An interesting adipokine, is apelin that has been found to be markedly up-regulated in hepatic cirrhosis [77,78]. It’s overexpression was found in HSCs and the use of an antagonist of the apelin receptor attenuates both angiogenesis and hepatic fibrosis [78].
Conclusion
Hepatic angiogenesis is a hallmark of CLDs which aggravates fibrosis progression. Vascular remodeling contributing to capillarization of the sinusoids with generation of intrahepatic shunts characterizes hepatic angiogenesis. HSCs may constitute a crossroad at the interaction between hepatic inflammation, fibrosis and angiogenesis. Adipokines as agents with the wide spectrum of activity may influence not only fibrosis, inflammation and steatosis but also angiogenesis. Serum levels of some of the adipokines reflect the intensity of liver angiogenesis in CLDs. Further studies are necessary to better determine the role of adipokines in new blood vessel formation in CLDs.
References
- Chaparro M, Sanz-Cameno P, Trapero-Marugan M, Garcia-Buey L, Moreno-Otero R. Mechanisms of angiogenesis in chronic inflammatory liver disease. Ann Hepatol. 2007; 6: 208-213.
- Kukla M. Angiogenesis: a phenomenon which aggravates chronic liver disease progression. Hepatol Int. 2013; 7: 4-12.
- Medina J, Arroyo AG, Sanchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. Hepatology. 2004; 39: 1185-1195.
- Kukla M, Gabriel A, Waluga M, Budak R, Mazur W. Angiogenesis in chronic viral hepatitis. Gastroenterol Pol. 2009; 16: 304-309.
- Amarapurkar AD, Amarapurkar DN, Vibhav S, Patel ND. Angiogenesis in chronic liver disease. Ann Hepatol. 2007; 6: 170-173.
- Valfre di Bonzo L, Novo E, Cannito S, Busletta C, Paternostro C, Povero D, et al. Angiogenesis and liver fibrogenesis. Histol Histopathol. 2009; 24: 1323-1341.
- Novo E, Povero D, Busletta C, Paternostro C, di Bonzo LV, Cannito S, et al. The biphasic nature of hypoxia-induced directional migration of activated human hepatic stellate cells. J Pathol. 2012; 226: 588-597.
- Paternostro C, David E, Novo E, Parola M. Hypoxia, angiogenesis and liver fibrogenesis in the progression of chronic liver diseases. World J Gastroenterol. 2010; 16: 281-288.
- Sanz-Cameno P, Trapero-Marugan M, Chaparro M, Jones EA, Moreno-Otero R. Angiogenesis: from chronic liver inflammation to hepatocellular carcinoma. J Oncol. 2010; 272170.
- Yang Y, Sun M, Wang L, Jiao B. HIFs, angiogenesis, and cancer. J Cell Biochem. 2013; 114: 967-974.
- Ciupinska-Kajor M, Hartleb M, Kajor M, Kukla M, Wylezol M, Lange D, et al. Hepatic angiogenesis and fibrosis are common features in morbidly obese patients. Hepatol Int. 2013; 7: 233-240.
- Kukla M, Gabriel A, Sabat D, Liszka L, Wilk M, Petelenz M, et al. Association between liver steatosis and angiogenesis in chronic hepatitis C. Pol J Pathol. 2010; 61: 154-160.
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003; 9: 677-684.
- Fernandez M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009; 50: 604-620.
- Shimizu I. Antifibrogenic therapies in chronic HCV infection. Curr Drug Targets Infect Disord. 2001; 1: 227-240.
- McCuskey RS, Reilly FD. Hepatic microvasculature: dynamic structure and its regulation. Semin Liver Dis. 1993; 13: 1-12.
- Camenisch G, Pisabarro MT, Sherman D, Kowalski J, Nagel M, Hass P, et al. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J Biol Chem. 2002; 277: 17281-17290.
- Novo E, Cannito S, Zamara E, Valfre di Bonzo L, Caligiuri A, Cravanzola C, et al. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am J Pathol. 2007; 170: 1942-1953.
- Huebert RC, Vasdev MM, Shergill U, Das A, Huang BQ, Charlton MR, et al. Aquaporin-1 facilitates angiogenic invasion in the pathological neovasculature that accompanies cirrhosis. Hepatology. 2010; 52: 238-248.
- Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009; 50: 957-969.
- Bertolani C, Marra F. The role of adipokines in liver fibrosis. Pathophysiology. 2008; 15: 91-101.
- Aleffi S, Petrai I, Bertolani C, Parola M, Colombatto S, Novo E, et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005; 42: 1339-1348.
- Anagnostoulis S, Karayiannakis AJ, Lambropoulou M, Efthimiadou A, Polychronidis A, Simopoulos C. Human leptin induces angiogenesis in vivo. Cytokine. 2008; 42: 353-357.
- Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res. 2008; 78: 356-365.
- Kaur J, Adya R, Tan BK, Chen J, Randeva HS. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun. 2010; 391: 1762-1768.
- Robertson SA, Rae CJ, Graham A. Induction of angiogenesis by murine resistin: putative role of PI3-kinase and NO-dependent pathways. Regul Pept. 2009; 152: 41-47.
- Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci USA. 2004; 101: 2476-2481.
- Fu BD, Yamawaki H, Okada M, Hara Y. Vaspin can not inhibit TNF-alpha-induced inflammation of human umbilical vein endothelial cells. J Vet Med Sci. 2009; 71: 1201-1207.
- Tsochatzis E, Papatheodoridis GV, Archimandritis AJ. The evolving role of leptin and adiponectin in chronic liver diseases. Am J Gastroenterol. 2006; 101: 2629-2640.
- Aleffi S, Petrai I, Bertolani C, Parola M, Colombatto S, Novo E, et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005; 42: 1339-1348.
- Kitade M, Yoshiji H, Kojima H, Ikenaka Y, Noguchi R, Kaji K, et al. Leptin-mediated neovascularization is a prerequisite for progression of nonalcoholic steatohepatitis in rats. Hepatology. 2006; 44: 983-991.
- Friedman SL. Transcriptional regulation of stellate cell activation. J Gastroenterol Hepatol. 2006; 21 Suppl 3: S79-83.
- Uygun A, Kadayifci A, Yesilova Z, Erdil A, Yaman H, Saka M, et al. Serum leptin levels in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2000; 95: 3584-3589.
- Kukla M, Mazur W, Buldak RJ, Zwirska-Korczala K. Potential role of leptin, adiponectin and three novel adipokines--visfatin, chemerin and vaspin--in chronic hepatitis. Mol Med. 2011; 17: 1397-1410.
- Testa R, Franceschini R, Giannini E, Cataldi A, Botta F, Fasoli A, et al. Serum leptin levels in patients with viral chronic hepatitis or liver cirrhosis. J Hepatol. 2000; 33: 33-37.
- Aleffi S, Navari N, Delogu W, Galastri S, Novo E, Rombouts K, et al. Mammalian target of rapamycin mediates the angiogenic effects of leptin in human hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2011; 301: G210-219.
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010; 140: 900-917.
- Suganami E, Takagi H, Ohashi H, Suzuma K, Suzuma I, Oh H, et al. Leptin stimulates ischemia-induced retinal neovascularization: possible role of vascular endothelial growth factor expressed in retinal endothelial cells. Diabetes. 2004; 53: 2443-2448.
- Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci USA. 2001; 98: 6390-6395.
- Grosfeld A, Zilberfarb V, Turban S, Andre J, Guerre-Millo M, Issad T. Hypoxia increases leptin expression in human PAZ6 adipose cells. Diabetologia. 2002; 45: 527-530.
- Zwirska-Korczala K, Kukla M, Ziolkowski A, Janczewska-Kazek E, Berdowska A, Sitkiewicz A, et al. Leptin, neopterin and hepatocyte growth factor as markers of fibrosis and inflammatory activity in chronic hepatitis C. Exp Clin Hep. 2005; 1: OR60-65.
- Piche T, Vandenbos F, Abakar-Mahamat A, Vanbiervliet G, Barjoan EM, Calle G, et al. The severity of liver fibrosis is associated with high leptin levels in chronic hepatitis C. J Viral Hepat. 2004; 11: 91-96.
- Shimizu H, Kakizaki S, Tsuchiya T, Nagamine T, Takagi H, Takayama H, et al. An increase of circulating leptin in patients with liver cirrhosis. Int J Obes Relat Metab Disord. 1998; 22: 1234-1238.
- McIlroy M, O'Rourke M, McKeown SR, Hirst DG, Robson T. Pericytes influence endothelial cell growth characteristics: role of plasminogen activator inhibitor type 1 (PAI-1). Cardiovasc Res. 2006; 69: 207-217.
- Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, et al. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med. 1996; 2: 800-803.
- Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, et al. Insulin resistance is associated with chronic hepatitis C infection and fibrosis progression. Gastroenterology. 2003; 125: 1695-1704.
- Bertolani C, Marra F. Role of adipocytokines in hepatic fibrosis. Curr Pharm Des. 2010; 16: 1929-1940.
- Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004; 40: 46-54.
- Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, et al. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005; 54: 117-121.
- Musso G, Gambino R, Biroli G, Carello M, Faga E, Pacini G, et al. Hypoadiponectinemia predicts the severity of hepatic fibrosis and pancreatic Beta-cell dysfunction in nondiabetic nonobese patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2005; 100: 2438-2446.
- Aygun C, Senturk O, Hulagu S, Uraz S, Celebi A, Konduk T, et al. Serum levels of hepatoprotective peptide adiponectin in non-alcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2006; 18: 175-180.
- Jiang LL, Li L, Hong XF, Li YM, Zhang BL. Patients with nonalcoholic fatty liver disease display increased serum resistin levels and decreased adiponectin levels. Eur J Gastroenterol Hepatol. 2009; 21: 662-666.
- Durante-Mangoni E, Zampino R, Marrone A, Tripodi MF, Rinaldi L, Restivo L, et al. Hepatic steatosis and insulin resistance are associated with serum imbalance of adiponectin/tumour necrosis factor-alpha in chronic hepatitis C patients. Aliment Pharmacol Ther. 2006; 24: 1349-1357.
- Liu CJ, Chen PJ, Jeng YM, Huang WL, Yang WS, Lai MY, et al. Serum adiponectin correlates with viral characteristics but not with histologic features in patients with chronic hepatitis C. J Hepatol. 2005; 43: 235-242.
- Handy JA, Saxena NK, Fu P, Lin S, Mells JE, Gupta NA, et al. Adiponectin activation of AMPK disrupts leptin-mediated hepatic fibrosis via Suppressors of Cytokine signaling (SOCS-3). J Cell Biochem. 2010; 110: 1195-1207.
- Caligiuri A, Bertolani C, Guerra CT, Aleffi S, Galastri S, Trappoliere M, et al. Adenosine monophosphateactivated protein kinase modulates the activated phenotype of hepatic stellate cells. Hepatology. 2008; 47: 668-676.
- Kukla M, Zwirska-Korczala K, Gabriel A, Waluga M, Warakomska I, Berdowska A, et al. Visfatin serum levels in chronic hepatitis C patients. J Viral Hepat. 2010; 17: 254-260.
- Akbal E, Kocak E, Tas A, Yuksel E, Koklu S. Visfatin levels in nonalcoholic fatty liver disease. J Clin Lab Anal. 2012; 26: 115-119.
- Aller R, de Luis DA, Izaola O, Sagrado MG, Conde R, Velasco MC, et al. Influence of visfatin on histopathological changes of non-alcoholic fatty liver disease. Dig Dis Sci. 2009; 54: 1772-1777.
- Kukla M, Ciupinska-Kajor M, Kajor M, Wylezol M, Zwirska-Korczala K, Hartleb M, et al. Liver visfatin expression in morbidly obese patients with nonalcoholic fatty liver disease undergoing bariatric surgery. Pol J Pathol. 2010; 61: 147-153.
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000; 407: 242-248.
- Kukla M, Berdowska A, Gabriel A, Sawczyn T, Mazur W, Sobala-Szczygiel B, et al. Association between hepatic angiogenesis and serum adipokine profile in non-obese chronic hepatitis C patients. Pol J Pathol. 2011; 62: 218-228.
- Kukla M, Zwirska-Korczala K, Gabriel A, Waluga M, Warakomska I, Szczygiel B, et al. Chemerin, vaspin and insulin resistance in chronic hepatitis C. J Viral Hepat. 2010; 17: 661-667.
- Kukla M, Zwirska-Korczala K, Hartleb M, Waluga M, Chwist A, Kajor M, et al. Serum chemerin and vaspin in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2010; 45: 235-242.
- Bozaoglu K, Curran JE, Stocker CJ, Zaibi MS, Segal D, Konstantopoulos N, et al. Chemerin, a novel adipokine in the regulation of angiogenesis. J Clin Endocrinol Metab. 2010; 95: 2476-2485.
- Di Simone N, Di Nicuolo F, Sanguinetti M, Castellani R, D'Asta M, Caforio L, et al. Resistin regulates human choriocarcinoma cell invasive behaviour and endothelial cell angiogenic processes. J Endocrin. 2006; 189: 691-699.
- Verma S, Li SH, Wang CH, Fedak PWM, Li RK, Weisel RD, et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003; 108: 736-740.
- Mu H, Ohashi R, Yan S, Chai H, Yang H, Lin P, et al. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res. 2006; 70: 146-157.
- Bertolani C, Sancho-Bru P, Failli P, Bataller R, Aleffi S, DeFranco R, et al. Resistin as an intrahepatic cytokine. Over expression during chronic injury and induction of iroinflammatory actions in hepatic stellate cells. Am J Pathol. 2006; 169: 2042-2053.
- Tsochatzis E, Papatheodoridis GV, Hadziyannis E, Georgiou A, Kafiri G, Tiniakos DG, et al. Serum adipokine levels in chronic liver diseases: association of resistin levels with fibrosis severity. Scan J Gastroenterol. 2008; 43: 1128-1136.
- Kukla M, Waluga M, Sawczyn T, Berdowska A, Kajor M, Boryczka G, et al. Serum vaspin may be a good indicator of fibrosis in chronic hepatitis C and is not altered by antiviral therapy. Pol J Pathol. 2012; 63: 213-220.
- Aktas B, Yilmaz Y, Eren F, Yonal O, Kurt R, Alahdab YO, et al. Serum levels of vaspin, obestatin, and apelin-36 in patients with nonalcoholic fatty liver disease. Metabolism. 2011; 60: 544-549.
- Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci USA. 2005; 102: 10610-10615.
- Jung CH, Lee MJ, Kang YM, Lee YL, Yoon HK, Kang SW, et al. Vaspin inhibits cytokine-induced nuclear factor-kappa B activation and adhesion molecule expression via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc Diabetol. 2014; 13: 41.
- Phalitakul S, Okada M, Hara Y, Yamawaki H. Vaspin prevents methylglyoxal-induced apoptosis in human vascular endothelial cells by inhibiting reactive oxygen species generation. Acta Physiol (Oxf). 2013; 209: 212-219.
- Liu S, Dong Y, Wang T, Zhao S, Yang K, Chen X, et al. Vaspin inhibited proinflammatory cytokine induced activation of nuclear factor-kappa B and its downstream molecules in human endothelial EA.hy926 cells. Diabetes Res Clin Pract. 2014; 103: 482-488.
- Kasai A, Shintani N, Oda M, Kakuda M, Hashimoto H, Matsuda T, et al. Apelin is a novel angiogenic factor in retinal endothelial cells. Biochem Biophys Res Commun. 2004; 325: 395-400.
- Principe A, Melgar-Lesmes P, Fernandez-Varo G, del Arbol LR, Ros J, Morales-Ruiz M, et al. The hepatic apelin system: a new therapeutic target for liver disease. Hepatology. 2008; 48: 1193-1201.