Mini Review
Austin Biom and Biostat. 2014;1(1): 3.
Statistical Learning in a Multisensory World
Lihan Chen*
Department of Psychology, Peking University, China
*Corresponding author: Lihan Chen, Department of Psychology, Peking University, China.
Received: September 04, 2014; Accepted: September 18, 2014; Published: September 19, 2014
Abstract
In this mini-review, the general paradigm used for Statistical Learning (SL) was traced and the main theoretical debate of brain modular vs. centralized processing was introduced, mainly from the evidence of multisensory interaction that is documented in the literature. Moreover, the time course of SL has been concisely delineated. Finally, a survey of the neuronal exploration of SL was given, although the endeavor in revealing neural mechanism is insufficient so far.
Keywords: Statistical learning; Cross-modality; Development; Modular
Introduction
As active learners, we rely on a combination of experience-independent and experience-dependent mechanisms to extract information from the environment. Statistical Learning (SL) has been studied as a mechanism by which people automatically discover patterns in the environment through experience. SL starts remarkably early in the progress of human life-span development. For instance, 8-month-old infants are capable of extracting serial-order information after only 2 min of listening experience [1].
It is the brain's capacity to detect statistical regularities in the environment, by operating complex perceptual and cognitive manipulations to obtain object recognition [2,3], event identification [4-7], and even language acquisition [8,9]. The information of the sensory events is usually presented sequentially, given by a specific sensory modality or a combination of modalities (such as auditory and visual modalities). The efficiency of SL differs among different sensory modalities. In general, auditory modality displays a quantitative learning advantage compared with vision and touch [4,5]. The disparities in sensory processing have been commonly recognized as sensory dominance ever since 1980 [10]. To substantiate the sensory dominance/difference in SL, a paradigm of artificial grammar has been developed and applied extensively in a large body of experimental explorations. A typical procedure of experiment goes as follows: observers are required to make 'match' or 'mismatch' discrimination of the two presented stimuli sequences (both are visual, auditory or tactile sequences), in which the presentation orders of spatial locations for a visual square, or the pitches of an auditory sequence containing multiple beeps were aligned by the given predefined grammar. Using the artificial grammar protocol, a number of studies have demonstrated that the auditory modality appears to have an advantage in the processing of sequential input, including low-level temporal processing tasks and pattern or rhythm discrimination, while touch modality is adept at processing both sequential and spatial input, though it is not at the same level of proficiency as either audition or vision [4-6,11].
Supra-modal or modular processing?
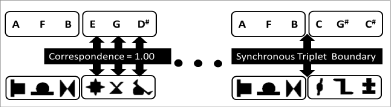
A central debate in SL concerns whether learners encode the regularities with an abstract or stimulus-specific representation. Modular theories hypothesize that SL is accomplished by mechanisms particular to the domain in question [12-14]. However, it has been widely observed various domains and many sorts of species that could attune to probabilistic patterns in the environment [15-18] suggesting a supra-modal representation of SL from phylogenetic perspective. To resolve this debate, people usually investigate whether there is a transfer of learning (benefits) from one stimulus set to another, independent of perceptual features of the stimuli or the sensory modality. For example, whether there is a transfer from the dimension (visual shapes) to another dimension (auditory pitch), namely cross-modally. Alternatively, one may examine how the cross-modal relationships influence simultaneous learning of multimodal input streams. For the latter, Seitz et al. [19] found statistical learning is a modality-independence process in which observers could extract concurrent, multiple (auditory vs. visual) statistical patterns equally, supporting the view of modular (modality-independence) processing [19]. In contrast, Mitchel and Weiss [6] presented both auditory and visual streams simultaneously or asynchronously, with variable predictability (transitional probabilities) between audio and visual elements and asked the observers to segment the boundaries of the audio and visual triplets (Figure 1). The results suggest that learners were able to extract multiple statistical regularities across modalities provided that there is some degree of cross-modal coherence [6], favoring a supra-modal abstract representation.
Figure 1: Stimuli pattern adapted from Mitchel and Weiss [6]. Two sequences of auditory music tones and artificial visual figures were presented. The correspondence of each tone and figure is manipulated to the predefined artificial grammar.
Most recently, Mitchel et al. accounted (auditory) statistical learning with modality-interactive mechanism by employing the perception of McGurk illusion [20]. In McGurk task, concurrent incongruous visual information (lip movements) biases the auditory perception of speech. Mitchel et al. [20] demonstrated the perception of audiovisual illusory syllables, acquiring from statistical leanring, altered the auditory stream structure and thus facilitated participants' ability to segment the speech stream.
Developmental evidence and individual approaches
To delineate the time course of development, Hupp and Sloutsky [21] investigated the development of (cross-modal) transfer between 8 and 16 months of age 8- and 16-month-olds were trained to attend to the end of a visual stimuli sequence (in a same modality). They were then tested on novel visual sequences. Results indicated transfer of learning, with both groups changing baseline preferences (i.e., looking times) as a result of training. In a separate experiment, participants were trained to attend to the end of a visual sequence while were tested on an auditory sequence. In difference to experiment 1, only older observers showed transfer of learning by changing baseline preferences (i.e., looking time at the targets). It suggest that the the generalization of learning, especially cross-modally, is improved within a critical period between 8 and 16 months [21].
Recently, research on SL has focused on individual differences by recruiting atypical developing groups. Mayo and Eigsti [22] found that High Functioning Children with Autism (HFA) and Typical Developing (TD) groups were equally able to implicitly learn transitional probabilities from a lengthy stimulus stream, and the task performance was not strongly associated with their current language abilities [22].
Neural evidence for SL
In contrast to the ample behavioral evidence of SL, the neural exploration of SL is relatively scarce. To determine the time course and neural processes involved in online word segmentation and SL of visual sequence, Abla and Okanoya [23] recorded Event-Related Potentials (ERPs) while participants were exposed to continuous sequences with elements organized into shape-words randomly connected to each other. The participants were divided into two groups (high and low learners) based on their behavioral performance (three sessions of training, with each session of 6 minutes). Grand-averaged ERPs showed that triplet-onset (the initial shapes of shape-words) elicited larger N400 amplitudes than did middle and final shapes embedded in continuous streams during the early learning sessions of high learners, but no triplet-onset effect was found among low learners. The results suggested that the N400 effect severed as a neural signature for online segmentation of the visual sequence and the degree of SL [23].
Paraskevopoulos et al. [24] assessed the effect of musical training in SL of tone sequences using Magneto Encephalography (MEG). MEG recordings were used to investigate the neural and functional correlates of the pre-attentive ability for detection of deviance, from a statistically learned tone sequence. Both normal and musicians groups revealed a significant difference between the standards and the deviants in the response of P50. However, this difference was significantly larger for the group of musicians. The results indicates that a long term exercise can enhance the ability of the auditory cortex to discriminate new auditory events from previously learned ones according to transitional probabilities [24].
Conclusion
In conclusion, statistical learning is of primary importance to humans as well as higher-order organisms and remains a vibrant topic in experimental psychology. The debate of 'modality-dependency' has not been fully resolved. The deep investigations into the underlying neural mechanism/representations of SL, especially from the lifespan development perspective [25], would help to capture as well as describe how efficiency human observers cope adaptively with the changing multisensory environment.
References
- Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month-old infants. Science. 1996; 274: 1926-1928.
- Fiser J, Aslin RN. Unsupervised statistical learning of higher-order spatial structures from visual scenes. Psychol Sci. 2001; 12: 499-504.
- Kim R, Seitz A, Feenstra H, Shams L. Testing assumptions of statistical learning: is it long-term and implicit? Neurosci Lett. 2009; 461: 145-149.
- Conway CM, Christiansen MH. Modality-constrained statistical learning of tactile, visual, and auditory sequences. J Exp Psychol Learn Mem Cogn. 2005; 31: 24-39.
- Conway CM, Christiansen MH. Statistical learning within and between modalities: pitting abstract against stimulus-specific representations. Psychol Sci. 2006; 17: 905-912.
- Mitchel AD, Weiss DJ. Learning across senses: cross-modal effects in multisensory statistical learning. J Exp Psychol Learn Mem Cogn. 2011; 37: 1081-1091.
- Saffran JR, Johnson EK, Aslin RN, Newport EL. Statistical learning of tone sequences by human infants and adults. Cognition. 1999; 70: 27-52.
- Hay JF, Pelucchi B, Graf Estes K, Saffran JR. Linking sounds to meanings: infant statistical learning in a natural language. Cogn Psychol. 2011; 63: 93-106.
- Lew-Williams C, Pelucchi B, Saffran JR. Isolated words enhance statistical language learning in infancy. Dev Sci. 2011; 14: 1323-1329.
- Welch RB, Warren DH. Immediate perceptual response to intersensory discrepancy. Psychol Bull. 1980; 88: 638-667.
- Mahar D, Mackenzie B, McNicol D. Modality-specific differences in the processing of spatially, temporally, and spatiotemporally distributed information. Perception. 1994; 23: 1369-1386.
- Chomsky N. Syntactic Structures. Mouton, The Hague. 1975.
- Fodor JA. The Modularity of Mind. MIT Press, Cambridge, MA. 1983.
- Fodor JA, Bever TG, Garrett MF. The Psychology of Language: An Introduction to Psycholinguistics and Generative Grammar. McGraw Hill, New York. 1974.
- Cordes S, King AP, Gallistel CR. Time left in the mouse. Behav Processes. 2007; 74: 142-151.
- Hasher L, Zacks RT. Automatic processing of fundamental information: the case of frequency of occurrence. Am Psychol. 1984; 39: 1372-1388.
- Kelly MH, Martin S. Domain-general abilities applied to domain-specific tasks: Sensitivity toprobabilities in perception, cognition, and language Lingua. 1994; 92: 105-140.
- Reber AS. Implicit Learning and Tacit Knowledge: an Essay on the Cognitive Unconscious. Oxford University Press, New York. 1993.
- Seitz AR, Kim R, van Wassenhove V, Shams L. Simultaneous and independent acquisition of multisensory and unisensory associations. Perception. 2007; 36: 1445-1453.
- Mitchel AD, Christiansen MH, Weiss DJ. Multimodal integration in statistical learning: evidence from the McGurk illusion. Front Psychol. 2014; 5: 407.
- Hupp JM, Sloutsky VM. Learning to learn: From within-modality to cross-modality transfer during infancy. J Exp Child Psychol. 2011; 110: 408-421.
- Mayo J, Eigsti IM. Brief report: a comparison of statistical learning in school-aged children with high functioning autism and typically developing peers. J Autism Dev Disord. 2012; 42: 2476-2485.
- Abla D, Okanoya K. Visual statistical learning of shape sequences: an ERP study. Neurosci Res. 2009; 64: 185-190.
- Paraskevopoulos E, Kuchenbuch A, Herholz SC, Pantev C. Statistical learning effects in musicians and non-musicians: an MEG study. Neuropsychologia. 2012; 50: 341-349.
- Daltrozzo J, Conway CM. Neurocognitive mechanisms of statistical-sequential learning: what do event-related potentials tell us? Front Hum Neurosci. 2014; 8: 437.