
Editorial
Austin J Biotechnol Bioeng. 2016; 3(2): 1061.
Nanocarriers for AKT siRNA Based Gene Therapy
Dubey P1 and Gopinath P1,2*
1Centre for Nanotechnology, Indian Institute of Technology Roorkee, India
2Department of Biotechnology, Indian Institute of Technology Roorkee, India
*Corresponding author: Gopinath P, Department of Biotechnology, Nanobiotechnology Laboratory, Indian Institute of Technology, Roorkee, Uttarakhand-247667, India
Received: April 27, 2016; Accepted: May 03, 2016; Published: May 05, 2016
Editorial
In the past few decades significant attention has drawn for gene silencing strategies based on RNA interference (RNAi)-mediated knock down of oncogene targeted cancer therapy [1]. RNAi refers to a method, where specific protein suppression could be achieved by delivery of double stranded short (20-25bp) interfering RNA (siRNA) [2]. However, the successful RNAi mediated gene therapy depends upon effective intracellular delivery of siRNA either as preformed siRNA or in conjugation with expressing plasmid vector and the efficient knock-down of oncogene transcripts [3].
Among various oncogenic targets, AKT⁄ Protein Kinase B (PKB) remains the central player in cell signaling pathways, altering cell survival and death [4]. Its activation leads to apoptotic resistance in cells, support cell survival, growth, and migration, energy metabolism and angiogenesis. It is evident from various studies that AKT perturbations plays an important part in tumorigenesis, [5] based on constitutive and increased expression of various AKT isoforms in diverse cancers, the inactivation of antagonists such as Phosphatase Tensin Homolog (PTEN), or mRNA over expression [6,7]. Its antiapoptotic action accounts for cell transforming ability and drug resistance in cancer cells against various chemotherapeutic agents [8]. Hence, AKT appears to play a pivotal role in the growth and tumor cells survival. Activation of Phosphatidylinositol 3-Kinase (PI3K)-/ AKT due to genetic alteration leads to chemotherapeutic insensitivity in diverse cancer preclinical and clinical trials [7,9]. Its dysregulation showed profound effect on the sensitivity of doxorubicin and 4-hydroxyl tamoxifen toward breast cancer chemotherapeutics [10]. Furthermore, characteristic AKT activation has been observed in various human tumor malignancies thus resulting in unfortunate predictive results [11,12]. Three AKT isoforms: AKT1⁄PKBα, AKT2⁄PKBβ, and AKT3⁄PKBc namely found in mammalian tissues, among which AKT1 and AKT2 has been found to be ubiquitously expressed in all type tissues observed and upregulated in various transformed tissues [5]. All these isoforms share amino acid homology closely and gets activated by PI3K-dependent pathway [2].
Consequently, AKT regulation possesses tremendous therapeutic attention and can be accomplished by successful delivering of AKTsiRNA. But for successful AKT-siRNA delivery, capable and safe nanocarrier is utmost necessity, and has appeared as major hurdle in siRNA based therapeutics. Though the RNAi is naturally occurring process in cells which provide all necessary components including the formation of RNA-Induced Silencing Complex (RISC) induces dicer endonuclease mediated cleavage of mRNA as showed in Figure 1a [13]. However the delivery of therapeutic siRNA is essential for induction of RNAi. Thus difficulties in the systemic delivery of siRNA to targeted tissues due to poor intracellular uptake, immunogenic response and limited blood stability hinders the siRNA therapeutics [14]. Additionally, delivery of siRNA to the target tumor site has been another hurdle in gene therapy. Furthermore, the bare siRNAs do not able to easily cross the cell membrane due to their negative charge and size [15].
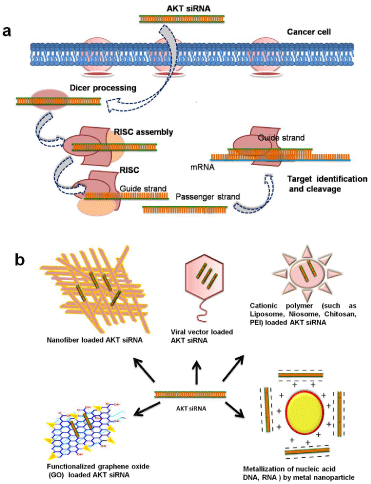
Figure 1: a) Schematic of oncogene silencing mechanism in cancer cell
assisted by AKT-siRNA, b) Various nanocarriers as vehicle for AKT-siRNA
delivery.
Therefore in order to augment the intrinsic therapeutic potency and delivery of siRNA, various nanocarrier-based targeted gene therapies have explored (Figure 1b) [16]. Among various nanocarriers, viral vectors have been explored primarily [17]. Despite providing high transfection efficiency, their applications were limited by immunotoxicity, chances of occurrence of mutagenicity and tumorigenesis. Considering the safety concern the non-viral vectors have been investigated such as various natural and synthetic polymers, cationic lipids and metal nanoparticles etc., [18]. Among which the cationic liposomes have exhibit hopeful results as delivery vector by protecting the siRNA against nucleases and evading the endosomal degradation [19,20]. Nevertheless, the use of liposomes was limited based on their reduced stability in physiological medium, short circulation life, and lack of controlled release system. Recently, a new class of liposomes known as niosomes is evolved as an alternate with enhanced rigidity, stability, biocompatibility, and high dispersive nature that avoid the blockage of vessels [21].
Among cationic natural polymers, the chitosan based nanocarrier has been successfully applied for Among cationic natural polymers, the chitosan based nanocarrier has been successfully applied for in vitro and in vivo gene delivery [22-25]. It is a mucoadhesive, biodegradable and biocompatible, cationic polysaccharide, however, its low transfection efficiency based on its poor buffering ability for intracellular endosomal escape remained the challenge. Studies have been done to further improve its transfection efficiency by grafting it with synthetic cationic polymer, the Polyethylenimine (PEI) (CHI-g-PEI) [26] which showed better cell viability in vitro, and in vivo with enhanced transfection efficiency on aerosol delivery [2,26-28]. and in vivo gene delivery [22-25]. It is a mucoadhesive, biodegradable and biocompatible, cationic polysaccharide, however, its low transfection efficiency based on its poor buffering ability for intracellular endosomal escape remained the challenge. Studies have been done to further improve its transfection efficiency by grafting it with synthetic cationic polymer, the Polyethylenimine (PEI) (CHI-g-PEI) [26] which showed better cell viability in vitro, and in vivo with enhanced transfection efficiency on aerosol delivery [2,26-28].
Therefore among synthetic polycationic polymers used for siRNA delivery, PEI take an important place based on its comparatively high gene transfection efficacy [29,30]. It is a water-soluble polymer with protonable amino groups, provides high cationic charge density at physiological pH. Various modifications of PEI with several ligands or coupling agents as cell-specific moieties are promising approaches to enhance the specificity, biocompatibility, and transfection. In physiological conditions, PEI remains protonated which allows it to form electrostatic complexes with nucleic acid molecules called as ‘polyplexes’ or nanoplexes which offers the shield for siRNA from nucleolytic enzyme degradation, promote efficient uptake via endocytosis and intracellular release through the phenomena called as ’proton sponge effect’ [27]. These polyplexes enter the cells via caveolae- or clathrin mediated pathways, where with the former route leads to efficient transfection. A study showed biodegradable nano-polymeric systems based on poly (ester amine) carrier based on Polycaprolactone (PCL) and PEI which showed impressive in vitro and in vivo gene delivery [30].
Recently, Graphene Oxide (GO) has been came in limelight based on its exceptional properties which leads to extensive investigation of GO for various applications including the drug delivery, biosensors, bioimaging, and gene therapy. The first study of GO as gene delivery vehicle was done by Liu and co-workers in combination with PEI, which was then tagged with EGFP plasmid DNA (pDNA) for intercellular gene transfection in HeLa cells. In another study Zhang and co-workers prepared PEI-grafted GO (GO– PEI) through covalent modification as an excellent nanocarrier for delivery of siRNA and drugs in vitro [31]. Recently the GO–PEI– PEG functionalized nanocarrier has been explored with an excellent physiological stability and solubility with low toxicity for delivering siRNA, CpG, STAT3, VEGF etc into cells [32-34].
Nanofibers have been widely explored for numerous applications, including antibacterial, cancer cell and tissue engineering field [35,36]. Recently the electrospun nanofibers were explored for their gene therapy potential [37]. As a template for nucleic acid delivery, nanofibers offers various advantageous features including the ease of production, their ECM-mimic behavior, feasibility of providing various properties by modifications, and their large surface area. It exhibits a great ability to control the release kinetics of gene vectors and enhance gene delivery efficiency [38,39].
Recently the concept of DNA metallization has been expanded for gene delivery. A study reported facile synthesis of pDNAtemplated silver nanoparticles (Ag NP), which could serve as a platform for effective gene delivery [40,41]. Compared to conventional nanocarriers, the metalized-pDNA offers numerous advantages, including providing appropriate size and surface charge, facile synthesis and minimal cytotoxicity, thus biocompatible nanomaterials for efficient gene delivery.
Though many studies are done for exploration of gene delivery nanocarriers, however the clinical translation of these nanocarriers is the major hurdle due to the non-specificity, cytotoxicity, biocompatibility and stability in physiological milieu emerged as critical bottleneck for siRNA therapeutics. Hence for successful siRNA delivery, safe and efficient gene delivery system is imperative.
Acknowledgment
Our sincere thanks to Science and Engineering Research Board (No. SR/FT/LS-57/2012), Government of India, for the financial support.
References
- Devi GR. siRNA-based approaches in cancer therapy. Cancer Gene Ther. 2006; 13: 819-829.
- Jere D, Jiang HL, Kim YK, Arote R, Choi YJ, Yun CH, et al. Chitosan-graft-polyethylenimine for Akt1 siRNA delivery to lung cancer cells. Int J Pharm. 2009; 378: 194-200.
- Kim WJ, Christensen LV, Jo S, Yockman JW, Jeong JH, Kim YH, et al. Cholesteryl Oligoarginine Delivering Vascular Endothelial Growth Factor siRNA Effectively Inhibits Tumor Growth in Colon Adenocarcinoma. Mol Ther. 2006; 14: 343-350.
- Lee MW, Kim DS, Lee JH, Lee BS, Lee SH, Jung HL, et al. Roles of AKT1 and AKT2 in non-small cell lung cancer cell survival, growth, and migration. Cancer Sci. 2011; 102: 1822-1828.
- Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004; 428: 332-337.
- Ma J, Sawai H, Ochi N, Matsuo Y, Xu D, Yasuda A, et al. PTEN regulates angiogenesis through PI3K/Akt/VEGF signaling pathway in human pancreatic cancer cells. Mol Cell Biochem. 2009; 331: 161-171.
- Dey JH, Bianchi F, Voshol J, Bonenfant D, Oakeley EJ, Hynes NE. Targeting Fibroblast Growth Factor Receptors Blocks PI3K/AKT Signaling, Induces Apoptosis, and Impairs Mammary Tumor Outgrowth and Metastasis. Cancer Res. 2010; 70: 4151-4162.
- Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001; 15: 1406-1418.
- Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004; 29: 233-242.
- Rajput S, Puvvada N, Kumar BNP, Sarkar S, Konar S, Bharti R, et al. Overcoming Akt Induced Therapeutic Resistance in Breast Cancer through siRNA and Thymoquinone Encapsulated Multilamellar Gold Niosomes. Mol. Pharmaceutics. 2015; 12: 4214-4225.
- Xu J, Zhou JY, Wei WZ, Wu GS. Activation of the Akt survival pathway contributes to TRAIL resistance in cancer cells. PLoS One. 2010; 5: e10226.
- Zhuang J, Hawkins SF, Glenn MA, Lin K, Johnson GG, Carter A, et al. Akt Is Activated in Chronic Lymphocytic Leukemia Cells and Delivers a pro-Survival Signal: The Therapeutic Potential of Akt Inhibition. Haematologica. 2010; 95: 110-118.
- Pei Y, Hancock PJ, Zhang H, Bartz R, Cherrin C, Innocent N, et al. Quantitative evaluation of siRNA delivery in vivo. RNA. 2010; 16: 2553-2563.
- Zhang Y, Satterlee A, Huang L. In vivo gene delivery by nonviral vectors: overcoming hurdles? Mol Ther. 2012; 20: 1298-1304.
- Pirollo KF, Rait A, Zhou Q, Hwang SH, Dagata JA, Zon G, et al. Materializing the potential of small interfering RNA via a tumor-targeting nanodelivery system. Cancer Res. 2007; 67: 2938-2943.
- Singha K, Namgung R, Kim WJ. Polymers in small-interfering RNA delivery. Nucleic Acid Ther. 2011; 21: 133-147.
- Sabbioni S, Callegari E, Manservigi M, Argnani R, Corallini A, Negrini M, et al. Use of herpes simplex virus type 1-based amplicon vector for delivery of small interfering RNA. Gene Ther. 2007; 14: 459-464.
- Posadas I, Guerra FJ, Ceña V. Nonviral vectors for the delivery of small interfering RNAs to the CNS. Nanomedicine (Lond). 2010; 5: 1219-1236.
- Verreault M, Bally MB. siRNA-Mediated Integrin-Linked Kinase Suppression: Nonspecific Effects of siRNA/cationic Liposome Complexes Trigger Changes in the Expression of Phosphorylated-AKT and mTOR Independently of ILK Silencing. Oligonucleotides. 2009; 19: 129-140.
- Meng Q, Xia C, Fang J, Rojanasakul Y, Jiang BH. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal. 2006; 18: 2262-2271.
- Kazi KM, Mandal AS, Biswas N, Guha A, Chatterjee S, Behera M, et al. Niosome: A future of targeted drug delivery systems. J Adv Pharm Technol Res. 2010; 1: 374-380.
- Chen M, Gao S, Dong M, Song J, Yang C, Howard KA, et al. Chitosan/siRNA nanoparticles encapsulated in PLGA nanofibers for siRNA delivery. ACS Nano. 2012; 6: 4835-4844.
- Lee JI, Ha KS, Yoo HS. Quantum-dot-assisted fluorescence resonance energy transfer approach for intracellular trafficking of chitosan/DNA complex. Acta Biomater. 2008; 4: 791-798.
- Jiang HL, Lim HT, Kim YK, Arote R, Shin JY, Kwon JT, et al. Chitosan-graft-spermine as a gene carrier in vitro and in vivo. Eur J Pharm Biopharm. 2011; 77: 36-42.
- Howard KA, Rahbek UL, Liu X, Damgaard CK, Glud SZ, Andersen MØ, et al. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol Ther. 2006; 14: 476-484.
- Jiang HL, Kim YK, Arote R, Nah JW, Cho MH, Choi YJ, et al. Chitosan-graft-polyethylenimine as a gene carrier. J Control Release. 2007; 117: 273-280.
- Günther M, Lipka J, Malek A, Gutsch D, Kreyling W, Aigner A. Polyethylenimines for RNAi-mediated gene targeting in vivo and siRNA delivery to the lung. Eur J Pharm Biopharm. 2011; 77: 438-449.
- Lei Wang, Weimin Wu, Jingshuai Wang, Jianjun Wang, Xiaowen Tong, Qiaoling Hu, et al. Highly efficient Gab2 siRNA delivery to ovarian cancer cells mediated by chitosan– polyethyleneimine nanoparticles. J Mater Chem B. 2016; 4: 273.
- Peng H, Yang H, Song L, Zhou Z, Sun J, Du Y, et al. Sustained delivery of siRNA/PEI complex from in situ forming hydrogels potently inhibits the proliferation of gastric cancer. J Exp Clin Cancer Res. 2016; 35: 57.
- Jere D, Arote R, Jiang HL, Kim YK, Cho MH, Cho CS. Biodegradable nano-polymeric system for efficient Akt1 siRNA delivery. J Nanosci Nanotechnol. 2010; 10: 3366-3369.
- Chen B, Liu M, Zhang L, Huang J, Yao J, Zhang Z. Polyethylenimine-Functionalized Graphene Oxide as an Efficient Gene Delivery Vector. J Mater Chem. 2011; 21: 7736.
- Paul A, Hasan A, Kindi HA, Gaharwar AK, Rao VT, Nikkhah M, et al. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano. 2014; 8: 8050-8062.
- Wang C, Wang X, Lu T, Liu F, Baofeng GB, Wen N, et al. Multi-functionalized graphene oxide complex as a plasmid delivery system for targeting hepatocellular carcinoma therapy. RSC Adv. 2016; 6: 22461.
- Tang L, Wang Y, Li J. The graphene/nucleic acid nanobiointerface. Chem Soc Rev. 2015; 44: 6954-6980.
- Dubey P, Bhushan B, Sachdev A, Matai I, Uday KS, Gopinath P. Silver-Nanoparticle-Incorporated Composite Nanofibers for Potential Wound-Dressing Applications. J Appl Polym Sci. 2015; 132.
- Dubey P, Gopinath P. Fabrication of electrospun poly(ethylene oxide)–poly(capro lactone) composite nanofibers for co-delivery of niclosamide and silver nanoparticles exhibits enhanced anti-cancer effects in vitro. J Mater Chem B. 2016; 4: 726-742.
- Sukumar UK, Packirisamy G. Bioactive Core-Shell Nanofiber Hybrid Scaffold for Efficient Suicide Gene Transfection and Subsequent Time Resolved Delivery of Prodrug for Anticancer Therapy. ACS Appl Mater Interfaces. 2015; 7: 18717-18731.
- Lee S, Jin G, Jang JH. Electrospun nanofibers as versatile interfaces for efficient gene delivery. J Biol Eng. 2014; 8: 30.
- Rujitanaroj PO, Wang YC, Wang J, Chew SY. Nanofiber-mediated controlled release of siRNA complexes for long term gene-silencing applications. Biomaterials. 2011; 32: 5915-5923.
- Tao Y, Ju E, Ren J, Qu X. Metallization of plasmid DNA for efficient gene delivery. Chem Commun (Camb). 2013; 49: 9791-9793.
- Gopinath P, Gogoi SK, Chattopadhyay A, Ghosh SS. Implications of silver nanoparticle induced cell apoptosis for in vitro gene therapy. Nanotechnology. 2008; 19: 075104.