
Special Article - Melanoma
Ann Carcinog. 2016; 1(1): 1006.
New Standard Therapeutics Approach at Metastatic Melanoma; Braf Mutated Patient Treated with Immunotherapy with Pembrolizumab
Chistian Patricio CL¹*, Raquel GC², Prieto AV³ and Riverol OL¹
¹Internal Medicine Specialist, Medical Oncology Specialist in American British Cowdray Medical Center, Mexico
²Medical Oncology Specialist, Director Cancer Center American British Cowdray Medical Center, Mexico
³Internal Medicine Specialist, Medical Hematology, American British Cowdray Medical Center, Mexico
*Corresponding author: Camacho-Limas Chistian Patricio, Cancer Center, American British Cowdray Medical Center, Mexico City, Alvaro Obregon, Las Americas, Sur 138, Mexico
Received: November 14, 2016; Accepted: December 14, 2016; Published: December 16, 2016
Abstract
Melanoma (MEL) represents a very important public health cause of cancer incidence, in according to SEER statistics since 1975 the incidence grew of 7.89/100,00hbs to 24.02/100,000 has at 2013. MEL accounts for the sixth cause of all cancer causes worldwide. In a metastatic stage clinical approach it is necessary to know BRAF and KIT mutation status as first therapeutic strategy based on NCCN guidelines recommendations. Now, a new approach is based on immuno-oncology strategy as standard of treatment (according to tumor expression of PD-L1, determined by a validated method).
Here we present a 58 years old female with metastatic melanoma showing an overall survival of 35 months after treatment with targeted therapy (dabrafenib/ trametinib) to which progressed. She was treated with Pembrolizumab (a PD-1 inhibitorm A b) having a very good response. Here we show a brief review of the evidence underlining the treatment of this tumor with immuno-therapy and we concluded that the new paradigm in precision oncology is a reality in melanoma metastatic stage treatment.
Keywords: Chemotherapy; BRAF; Immuno-oncology; CTLA-4 inhibitors; PD-L1 and PD-1 blockade
Case Report
A 58 years old female Ashkenazy Jewish, with family history of lung and breast cancer, past smoke history with no other relevant medical record. She was diagnosed with malignant melanoma on December, 19th 2013 after skin biopsy of her right arm, the extension imaging studies showed tumoral activity at central nervous system (multiple metastatic cortical deposits) by magnetic resonance (Figure1).
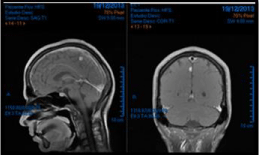
Figure 1: This imaging studies showed tumoral activity at central nervous system (multiple metastatic cortical deposits) by magnetic resonance.
The PET-CT with 5 FDG shows tumoral activity supradiaphragmatic lymphadenopathy, pulmonary nodules, muscle implants and right tibia injury; these findings are associated with increased metabolic activity in relation to the known primary secondary deposits (Figure 2).
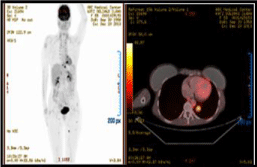
Figure 2: The PET-CT with 5 FDG shows tumoral activity supradiaphragmatic lymphadenopathy, pulmonary nodules, muscle implants and right tibia injury; these
findings are associated with increased metabolic activity in relation to the known primary secondary deposits.
The genetic analysis of skin biopsy results on BRAF mutation (V600E). She had had a good performance score and no medical counter-indication for treatment with trametinib with dabrafenib combo, started on January, 8th/2014 with further partial response showed by PET-CT ion April/2014 (Figure 3).
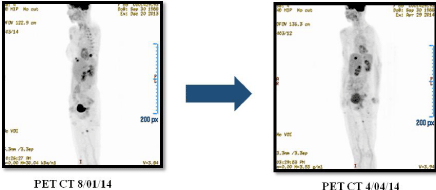
Figure 3: The genetic analysis of skin biopsy results on BRAF mutation (V600E).
Later, the patient was evaluated on October, 3rd,/2014, resulting with a stable disease after evaluation of the response with IRM and PET CT with FDG, which showed stable disease in NSC (Figure 4).
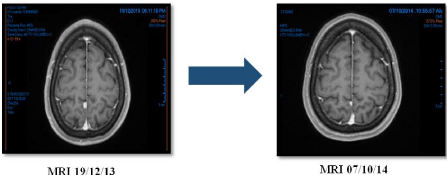
Figure 4: Resulting with a stable disease after evaluation of the response with IRM and PET CT with FDG, which showed stable disease in NSC.
On 2015, the patient coursed with unacceptable toxicity, mucositis grade 3-4 and diarrhea with rash, a new PET-CT on November, 2nd, 2015 showed disease progression, the toxicity was intolerable and she was treated on November, 27th 2015 with Ipilimumab (3mg/kg), 4 applications (Figure 5).

Figure 5: The patient coursed with unacceptable toxicity, mucositis grade 3-4 and diarrhea with rash.
After Ipilimumab treatment, she was evaluated with PET CT 18 FDG, resulting in stable disease but high toxicity (Diarrhea, and weight loss, rash). We decided to start Pembrolizumab (2mg/kg/ q3w) on February, 24th, 2015 time later, she coursed with partial response again and tolerable toxicity. After 1 year of treatment she was evaluated resulting with partial response.
To By September 2016 she has 20 months of overall survival with acceptable toxicity, and excellent quality of life, good performance status and practically without any symptom; she was evaluated on February, 24th, 2016 with PET CT with FDG, whose results are showed in (Figure 6).
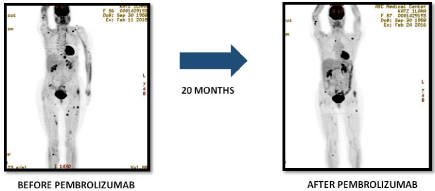
Figure 6: Variations in Pembrolizumab.
Literature review
Metastatic or recurrent melanoma treatment: Treatment for melanoma depends on the properties of the tumor and the stage at which the cancer is detected. If discovered early, the tumor may be removed surgically. In the case of advanced disease, the tumor is removed along with surrounding normal tissue and a sentinel lymph node. If a biopsy reveals that the cancer has spread to lymph nodes, treatments may include more extensive surgery, immunotherapy, targeted therapy, or clinical trial participation. Radiation therapy is sometimes used as well, depending upon properties of the tissue removed at surgery.
Since 2011, eight new drugs have been FDA approved for the treatment of melanoma, including four immunotherapies and four targeted therapies. The immunotherapy drugs are ipilimumab (Yervoy®), pembrolizumab (Keytruda®), nivolumab (Opdivo®), and talimogenelaherparepvec (T-VEC, Imlygic™). The first three drugs are checkpoint inhibitors that “take the brakes off” the immune system and enable it to fight cancer; the last is an oncolytic virus therapy.
The targeted therapies are vemurafenib (Zelboraf®), dabrafenib (Tafinlar®), trametinib (Mekinist®), and cobimetinib (Cotellic®). These drugs target common genetic mutations, such as the BRAFV600 mutation, found in a subset of melanoma patients.
Despite the recent FDA approvals of these drugs, some patients with advanced metastatic melanoma still have a significant risk of mortality. A substantial unmet need remains for new successful therapies in patients with this disease.
Chemotherapy
Chemotherapy based systemic treatment with was approved in 1970 based on overall response rates. Phase III trials, reported a 10 to 20% Overall Response Rate (ORR) and rarely observed complete responses did not show any effect on Overall Survival (OS) [1].
Phase III, clinical trial results of temozolomide, an oral drug member of the alkylating agent’s class, seemed to have effects similar to intravenous Dacarbazine; of primary endpoint of this trial was OS; However, the trial was designed to demonstrate superiority of temozolomide, endpoint not achieved, and was not powered to demonstrate no inferiority [2].
BRAF inhibitors
Currently, treatment of disseminated disease needs BRAF V600E mutation status determination in tumor tissue.
Approximately 50% of cutaneous melanomas have activating mutations in BRAF, this allows treatment with specific tyrosine kinase inhibitors, like vemurafenib or Dabrafenib. Both drugs are superior to classical chemotherapy in response rate, time to progression and overall survival, given orally qd: vemurafenib 960 mg every 12 hours and dabrafenib 150 mg every 12 hours [3].
MEK Inhibitors- like trametinib or cobimetinib are also useful to treat melanoma BRAF mutated. In a recent survey was shown that the combination of a BRAF and MEK inhibitors superior to them as monodrug, resulting in improved survival, so combination has become the standard treatment of BRAF mutated melanoma [4].
Immunotherapy
Different immunotherapy strategies have been described:
(a) Immunotherapy not customized like monoclonal antibodies targeting tumoral antigens (Anti CD19, CD20,)
(b) Enhancing cytokines (IL-2, IFNa) anti-tumor responses
(c) Immune check-point blocking antibodies (PD-1, PD-L1, CTLA-4)
CTLA-4: pre-clinical observations
The steps that lead to T-cell activation include antigen peptide presentation by Antigen Presenting Cells (APC) to T Cell Receptor (TCR) in the context of the appropriate Major Histocompatibility (MHC) complex molecule class (signal 1) and the commitment of a co-stimulator receiver (signal 2), all this mediated by CD28 interaction withCD80 / CD86 in APC. Other signals mediate by cytokines from APC or regulatory T cells may amplify or reduce the immune response (3 signal); T-cell activation also triggers pathways that eventually dampen the immune response.
The main regulatory molecule that inhibits T-cell response after activation is CTLA-4, which is normally stored in vesicles in the cytosol of T-cells and is released to the surface after antigen presentation, where compete with CD28 to link with CD80 /CD86, the net effect is T-cell activated cells reduction [5].
Ipilimumab
Ipilimumab is a fully human IgG1 monoclonal antibody that binds CTLA-4 receptor expressed in activated T cells.
Phase I and II clinical trials of Ipilimumab showed that it was biologically active and reasonably tolerable, these early studies have also established that patients with advanced melanoma had objective tumor regression.
Two phase III randomized trials with Ipilimumab were conducted in patients with advanced melanoma, the first study was conducted in patients with metastatic melanoma, where Ipilimumab was compared with a peptide vaccine known asgp100 as control group.
The objective response rate was low, but there was a statistically significant improvement in survival in patients receiving Ipilimumab.
Unlike chemotherapy, where tumor response tends to be shown within a few weeks, melanoma response after treatment with Ipilimumab often take weeks, or even months after completion of therapy.
Delayed responses to Ipilimumab or rapid progression followed by marked regression (pseudoprogresion) have also been reported, and are known as atypical responses.
The recognition of the marked differences in tumor response kinetics after anti-CTLA-4 therapy compared to chemotherapy and other immunotherapies has changed clinical practice.
These observations have led to alternative measurement rules to assess clinical response, known as immune response criteria in spite of existence of other validated criteria to assess objective response.
The Food and Drug Administration (FDA) approved Ipilimumab on March 2011 for patients with metastatic melanoma or unresectable disease [6].
PD-1: pre-clinical observations
The main way that immune system is negatively regulated peripherally (e.g. at sites of chronic infection or tumors) is by the PD1/PDL1 system, which could inactivate T cells targeted against tumor. PD-1 is a transmembrane protein expressed in T-cells surface and one of its functions is to protect the body from immunoreactivity It has two known ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC), which are expressed by CPA, macrophages, T and B cells and regulate activation of T cells. PD-L1 is expressed also by many tumors such as melanoma (Haile, et al. 2011). PD-1 may also interact with CD80, one of the ligands to CTLA-4, therefore, the expression of PD-1 can potentially strengthen the inhibitory signal of CTLA-4, PD-1 and the expression of PD-L1 are associated with energy and depletion of T cells, especially in the setting of chronic exposure to the Antigen that occurs during infection or malignancy Therefore, immunemodulation by the tumor within the tumor microenvironment can promote the survival of malignancy and decrease the ability of T cells cytotoxic to eradicate the tumor [7,8].
Blocking PD1 or PD-L1 antibody using antagonists have been shown to improve tumor responses in many murine tumor models and may be more effective in combination with anti-CTLA-4, these results provide a solid rationale for the clinical translation of blocking PD-1 / PD-L1 in patients with melanoma and other malignant tumors [9].
ANTI-PD-1 clinical results
The first antibody anti-PD-1 tested in patients with melanoma was MDX-1106, a fully human IgG4, now known as nivolumab.
This antibody blocks the interaction between PD-L1 and PD-1 and also the interaction between PD-1 and CD80; is found on B cells and macrophages whose normal function is to provide a coestimulating signal when engages CD28 in activated T cells.
Nivolumab was compared with the Dacarbazine in one randomized study with 418 patients with melanoma BRAF not mutated and without prior systemic treatment. The group that received nivolumab (n = 210) had a significantly better objective response (40% vs. 13.9%), survival at 1 year (72.9% versus 42.1%) and the progression free survival (5.1 vs. 2.2 months) compared with patients who received the dacarbazine [9].
Another antagonist antibody specific to compete with the interaction between PD-1 and PD-L1 and PD-L2, known as Pembrolizumab, is a fully human IgG4 antibody, was evaluated in 173 patients with melanoma non-resectable or metastatic who had disease progression after having received at least 2 doses of Ipilimumab subsequently were treated with Pembrolizumab to 2 mg / kg (N = 89) or 10 mg/kg (N = 84) [10,11]).
A randomized phase III study comparing two immune checkpoint inhibitors showed that Pembrolizumab, as compared with Ipilimumab, significantly prolonged Progression-Free Survival (PFS) and Overall Survival (OS) with fewer high-grade toxicities in patients with advanced melanoma. The results are published online on 20 April in The New England Journal of Medicine by Dr Caroline Robert as a corresponding author and colleagues and reported by Dr Antoni Ribas simultaneously at AACR Annual Meeting 2015 (18-22 April, Philadelphia, USA).
KEYNOTE-006 study was randomized, controlled, phase III study, in which 834 patients with advanced melanoma were assigned in a 1:1:1 ratio to receive Pembrolizumab at a dose of 10 mg per kilogram of body weight every 2 weeks or every 3 weeks or four doses of Ipilimumab at 3 mg per kilogram every 3 weeks. The study primary endpoints were PFS and OS.
Response was assessed at week 12 and every 6 weeks thereafter according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, on the basis of central radiologic review and immunerelated response criteria by investigator review.
Among enrolled patients, 65.8% had received no previous systemic treatment for advanced melanoma, 68.7% had an ECOG performance status of 0, 65.3% had stage M1c disease, and 32.4% had elevated Lactate Dehydrogenase (LDH) levels. BRAF V600 mutations were observed in 36.2% of patients, and of these, approximately 50% had received previous BRAF inhibitor treatment; 80.5% of patients had PD-L1–positive tissue samples.
The estimated 6-month PFS rates were 47.3% for Pembrolizumab every 2 weeks, 46.4% for Pembrolizumab every 3 weeks, and 26.5% for Ipilimumab (hazard ratio for disease progression, 0.58; p < 0.001 for both pembrolizumab regimens versus Ipilimumab).
Estimated 12-month survival rates were 74.1%, 68.4%, and 58.2%, respectively (hazard ratio for death for Pembrolizumab every 2 weeks, 0.63, p = 0.0005; hazard ratio for Pembrolizumab every 3 weeks, 0.69, p = 0.0036).
The response rate was improved with Pembrolizumab administered every 2 weeks (33.7%) and every 3 weeks (32.9%), as compared with ipilimumab (11.9%) (p < 0.001 for both comparisons). Responses were ongoing in 89.4%, 96.7%, and 87.9% of patients, respectively, after a median follow-up of 7.9 months. Efficacy was similar in the two Pembrolizumab groups.
The most common treatment-related adverse events of any grade occurring in the Pembrolizumab were fatigue (20.9% in the 2-week group and 19.1% in the 3-week group), diarrhea (16.9% and 14.4%, respectively), rash (14.7% and 13.4%, respectively), and pruritus (14.4% and 14.1%, respectively); all events were of grade 3 to 4 severity in less than 1% of patients, except diarrhea (2.5% and 1.1%, respectively).
The Food and Drug Administration (FDA) approved Pembrolizumab in September 2014 for the treatment of patients with advanced melanoma after ipilimumab failure/intolerance or progression under anti-BRAF therapy, currently there are about 85 clinical trials studying anti-PD-1 or anti-PD-L1 as monotherapy or in combination in patients with metastatic melanoma bladder cancer, lung cancer renal cancer and non-small cell lung cancer, with encouraging results [12].
In a recently study analysis was performed the somatic mutanomes and transcriptomes of pretreatment melanoma biopsies to identify factors that may influence innate sensitivity or resistance to anti-PD-1 therapy. We find that overall high mutational loads associate with improved survival, and tumors from responding patients are enriched for mutations in the DNA repair gene BRCA2. Innately resistant tumors display a transcriptional signature (referred to as the IPRES, or innate anti-PD-1 resistance), indicating concurrent up-expression of genes involved in the regulation of mesenchymal transition, cell adhesion, extracellular matrix remodeling, angiogenesis, and wound healing. Notably, mitogen-activated protein kinase (MAPK)-targeted therapy (MAPK inhibitor) induces similar signatures in melanoma, suggesting that a non-genomic form of MAPK inhibitor resistance mediates cross-resistance to anti-PD-1 therapy [13].
Radiotherapy
A question emerges for regulatory agencies, physicians and pharmaceutical companies: when a novel drug becomes a ‘blockbuster’, should specific safety studies address a priori the feasibility of concurrent radiotherapy, given the fact that a significant part of long term survivors in the metastatic setting will require SRT inhibitors Stereotactic Radiation (SRT) during the course of their disease or do we have to wait for isolated case reports of toxicities to raise this issue posterior such as in the recent case of B Rapidly Accelerated Fibrosarcoma (BRAF),the current work cannot provide definite answers but does suggest the combination is rather safe with all limitations due to the above-mentioned remarks.
Finally, this underscores the fact that new drugs leading to major survival improvements in metastatic patients will raise the question of safety for clinicians during tumor boards when SRT is secondarily required. Results of prospective long-term studies focusing on safety/efficacy endpoints in patients exposed to SRT and different immunotherapies are eagerly awaited [14].
Conclusion
Melanoma is one of the major cancer types for which new immune-based cancer treatments are currently available, with more in development.
Immunotherapy may be the next great hope for cancer treatment; it is likely that our best strategy to fight cancer will be to attack all sides. Clearly, different strategies have demonstrated benefit in different patient populations.
Despite rapid advances in biomarker-guided drug development in different tumor types, including melanoma, only a very small number of biomarkers have been identified. Recently, microRNAs (miRNAs) have emerged as a molecular regulator in the development and progression of melanoma. There is accumulating evidence suggesting the potential impact of circulating miRNAs as diagnostic and therapeutic markers in diseases. In addition, miRNAs have turned out to play important roles in drug-resistance mechanisms; suggesting their modulation as a potential approach to overcome chemo resistance.
It is important to explore novel prognostic markers in the management of patients with melanoma that could offer factors predictive in the treatment.
This case demonstrates the impact of current treatments in the management of metastatic melanoma, with an overall survival of more than 2 years with an excellent quality of life and a few side effects.
Today, immunotherapy has revolutionized the Oncology era offering more treatment opportunities to our patients; the effect of any of the aforementioned strategies combinated with traditional anti-cancer therapies is another field to be explored more in depth, as we have seen some benefit in terms of duration with cytokines and chemotherapy.
References
- Annual SEER incidence rates by race and sex (melanoma of the skin). Seer.cancer.gov/cgi-bin National Compresive Cancer Network. Guidelines V3.0 2016 Panel Members. Melanoma.
- Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000; 18: 158-166.
- Hellen D, Bignell GR, Cox Ch, Philip Stephens, Sarah Edkins, Sheila Clegg, et al. Mutation of the BRAF gene in human cancer. Nature. 2002; 417: 949-954.
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al, Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012; 380: 358-365.
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013; 39: 1-10.
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011; 364: 517-526.
- Tsai KK, Daud AI. The Role of Anti-PD-1/PD-L1 Agents in Melanoma. Drugs. 2015; 75: 563-575.
- Curran MA, Montalvo W, Yagita H, Allison J. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010; 107: 4275-4280.
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2010; 366: 2455-2465.
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014; 20: 5064-5074.
- Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. Association of Pembrolizumab with Tumor Response and Survival Among Patients with Advanced Melanoma. JAMA. 2016; 315: 1600-1609.
- Robert C, Schachter J, Long G, Arance A, Jacques Grob J, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. NEJM. 2015.
- Hugo W, Zaretsky JM. Cell. 165; 1: 35-44.
- Anker CJ, Ribas A, Grossmann AH, Chen X, Narra KK, Akerley W, et al. Severe liver and skin toxicity after radiation and vemurafenib in metastatic melanoma. J Clin Oncol. 2013; 31: 283-287.