
Special Article - Radiation Oncology
Ann Carcinog. 2017; 2(1): 1009.
Radiating on the Edge: A Rare Case of Adenoid Cystic Carcinoma of the Lacrimal Gland Treated by Eye-Sparing Surgery and Radiation Therapy
Oertel M¹*, Reinartz G¹, Barth PJ², Kittel C¹ and Eich HT¹
¹Department of Radiation Oncology, Albert-Schweitzer- Campus, Germany
²Department of Pathology, Albert-Schweitzer-Campus, Germany
*Corresponding author: Oertel Michael, Department of Radiation Oncology, Albert-Schweitzer-Campus 1 building A1, 48149 Muenster, Germany
Received: May 05, 2017; Accepted: May 30, 2017; Published: June 08, 2017
Abstract
About 1/3 of epithelial malignancies of the lacrimal gland are adenous cystic carcinoma making it a frequent malignant tumor only outstripped by lymphoma. Despite that fact, no standard treatment is established so far, but surgery and radiation therapy are used with good results, with the extent of surgery being a controversial issue. Concerning radiooncology, different doses and fractionation schemes have been used in the past. We present a case of an adenoid cystic carcinoma of the left lacrimal gland successfully treated by postoperative radiation therapy.
After a case presentation, we focus on a literature review on the role of radiation therapy in lacrimal adenoid cystic carcinoma.
Keywords: Orbital malignancies; Adenoid cystic carcinoma; Lacrimal tumor; Radiotherapy
Abbreviations
ACC: Adenoid Cystic Carcinoma; GTV: Gross Tumor Volume; IMRT: Intensity-Modulated Radiotherapy; PTV: Planning Target Volume
Case Presentation
Adenoid Cystic Carcinomas (ACC) represents the most frequent carcinoma of the lacrimal gland with a percentage of 32% [1]. Symptoms may vary and comprise loss of vision, propoptosis, diplopia, eye lid swelling, epiphora and pain [2,3].
Local therapy as operation or radiation therapy is complicated by the orbital anatomy involving the second, third and sixth cranial nerve and essential structures for ocular function as the lacrimal gland, external and internal muscles, lens and the retina [4]. The following case illustrates the interdisciplinary approach to a rare tumor entity and the considerations made for preserving the orbital structures functionally.
Patient
A 25-young gentleman was admitted to our institution with an impaired field of vision nasal-inferior on the left side. Ophthalmic examination showed a vision of 0.4 and a prominent tumor in the superior-temporal quadrant of the left orbita. In the initial MRI, a left bulb protrusion due to a sharp demarcated tumor in the left upper quadrant (2.8*2.5*2.1 cm) was noticed and thought to be a pleomorphic adenoma.
The patient underwent resection via a lateral, cranial transosseus orbitotomy. Intraoperatively, the tumor was found to be superficially and capsulated and was consequently removed, supposedly completely.
Pathological examination revealed an ACC of the lacrimal gland (Figure 1). A focal perineural invasion was found and the resection margins were infiltrated broadly by tumor cells. Subsequently, the carcinoma was staged as pT2, pNx, cMx, L0, V0, Pn1, R1 [5].
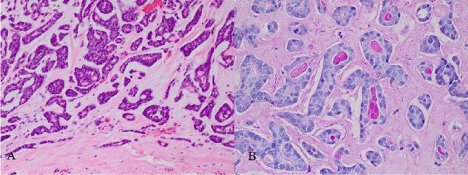
Figure 1: Histologically, small tumor cells with scant cytoplasm are arranged
in trabeculae and tubular groups surrounded in part by basement membranelike
material (A); the periodic acid-Schiff stains shows homogenous positive
material within lumina formed by the tumor (B).
Furthermore, a postoperative CT-scan demonstrated enlarged mediastinal and hilar lymph nodes from which a biopsy was taken via EBUS.
The incomplete resection warranted a more radical operation, so orbital enucleating was planned which was rejected by the patient.
After admission to Radiation Oncology, the patient was thoroughly counseled about postoperative radiotherapy and informed consent was granted. Patient underwent a planning CT of the head in a supine position with a thermoplastic mask minimizing possible movements. Organs at risk were delineated using the Eclipse™ treatment planning system (Version 10.0.45 Varian Medical Systems, Palo Alto, USA). To delineate the Planning Target Volume (PTV), image fusion with pre- and postoperative MRI was used. Preoperative Gross Tumor Volume (GTV) was delineated and extended by an area of postoperative changes in the upper lateral quadrant of orbit considering the presumed R1-resection medially. PTV contained this clinical target volume with a margin of 5 mm. Anatomically, the lacrimal fossa, lateral and superior portions of the orbita were included, consistent with the literature [6]. The PTV resembled an ellipsoid figure expanding into the eyeball and including the left optical nerve (Figure 2). Therefore, a main objective was to limit the radiation dose delivered to the second cranial nerve to preserve eyesight. We employed a two-step radiation plan in which the optical nerve was given a dose constraint of 55 Gy as maximum (Figure 3). First, 46.8 Gy were applied to the whole PTV and secondly, the second cranial nerve was blocked so dose maximum concentrated on the further tumor bed in the lateral orbita.
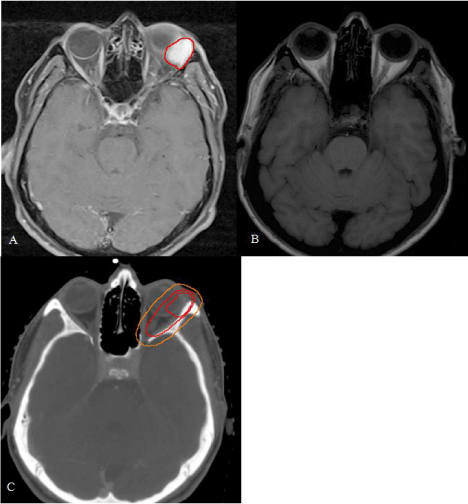
Figure 2: Delineation of PTV A: Preoperative MRI-images revealed a hyper
intense mass in the left superior orbita marked as GTV (red line). Notice
bulbus shifting to the medial orbital. B: Same position after surgery. Bulbus
has returned to its anatomical position. C: The MRI-images shown before are
image fused with planning-CT to mark GTV (internal red line). Clinical tumor
volume is contoured and involves postoperative changes and the possible
R1-site in the medial orbital (external red line). Finally, a safety margin of 0.5
mm is added to account for position uncertainties (PTV, orange line).
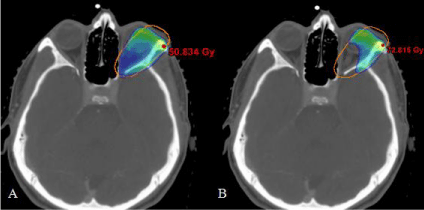
Figure 3: Radiating on the edge. 95 % isodoses as rainbow colour wash. A:
In a first step, 46.8 Gy are delivered to the PTV. B: Afterwards, the optical
nerve is excluded and radiation is continued up to 66.6 Gy.
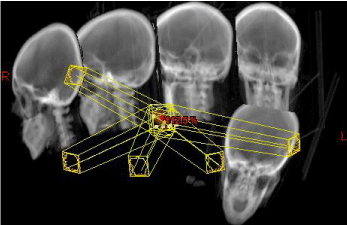
Figure 4: Digitally Reconstructed Radiograph: The image depicts the noncoplanar
field setup consisting of a five-field IMRT delivered in sliding-window
technique. The image shows the five gantry positions with an exemplary
multi-leaf-configuration.
Radiotherapy was performed by a True Beam linear accelerator (Varian Medical Systems) as a daily application of 1.8 Gy to a total dose of 66.6 Gy delivered with 6 MV photons. The treatment setup consisted of a five-field intensity-modulated radiotherapy IMRT in sliding window technique (Figure 4). In accordance with our dose constraints, doses were acceptable for all organs at risk (Table 1) except the left lens.
Dmin
Dmax
Dmean
Right lacrimal gland
2.9
5.6
4.1
Right lens
3.6
5.5
4.6
Left lens
51.8
71.5
66.5
Right eye
2.9
7.7
4.5
Left eye
22.8
72.2
62.1
Right optic nerve
7.6
21.0
14.5
Left optic nerve
47.9
54.3
50.1
Optic chiasm
9.4
43.0
22.8
Brain stem
0.8
22.0
10.0
Spinal cord
0.1
3.5
0.5
Right temporal lobe
0.6
23.3
6.4
Left temporal lobe
6.1
67.9
19.3
Right internal ear
0.9
4.0
1.8
Left internal ear
8.3
20.4
14.0
Mandible
0.1
19.4
1.0
Table 1: Doses for organs at risk: The table illustrates the minimal (Dmin), maximal (Dmax) and mean doses (Dmean) in Gy. The left lacrimal gland is missing as it has been removed surgically.
During treatment dose adequate side effects occurred as conjunctivitis with conjunctival injection, pruritus, epiphora and eyelid oedema which were addressed by moisturizing eye drops and eye drops containing sodium-hyaluronate. At the end of our treatment, periorbital hyperpigmentation and vertigo were present without evidence of intracranial pressure.
Therapy was further complicated by the fact that the lymphonodular biopsy described above was tested positively for mycobacterium tuberculosis. Therefore, a simultaneous therapy with isoniazid, rifampicin, ethambutoland pyrazinamide was started.
In an ophthalmological follow-up 1 week after radiation therapy a deterioration of vision was obvious with a decline to 0.16. A corneal erosion was present which was addressed by antibiotic and moisturizing eye ointment. Following RTOG-criteria, this constitutes grade 2 toxicity [7]. An MRI control 1 year after treatment demonstrated no clear evidence of tumor recurrence.
Discussion
ACC of the lacrimal gland is a rare disease with no established standard treatment. As most case studies only provide a limited amount of patients with rather anecdotal character, the state-of-the art is yet to be found.
Surgery is employed in nearly every reported case [2,3,8-14], although its extent may be doubtful. In a retrospective analysis of orbital epithelial malignancies, Polito et al. revealed a prolonged survival with less extensive surgery avoiding exenterating [12]. Additionally, Skinner et al. found no evidence for survival benefit in radical surgery when compared to eye sparing surgery [15]. Attention should be paid to the operational access as it may be contaminated with tumor cells [12].
Radiation therapy has proven to be effective in diminishing the risk for local recurrence despite no benefit in survival [1,15]. Of all treated patients, 54.8% underwent radiation therapy [1], mostly in a postoperative concept as this approach offers higher survival rates than a definitive treatment [16]. Disease control for incompletely resected ACC reaches 54% with radiation therapy [8] which is important as tumor cell positive margins and perineural invasion are frequent [17]. Doses vary between 30 and 74 Gy with a preference for total doses of at least 60 Gy [2,3,8-13], which has also been applied in our case-study. Data from the literature demonstrate a 5-year survival of 25-75% after radiotherapy [3,8,13,15]. It may be increased by the inclusion of intra-arterial chemotherapy which requires the availability of an intact lacrimal artery [14]. In contrast to that, radiotherapy is not limited by local anatomical boundaries.
As tumor control increases, toxicity attracts attention. A dry eye symptom as described in our case report is one of the most frequent side effects of orbital radiotherapy [10]. As mentioned above, one of our key concerns was to maintain the patient’s eyesight by reducing the dose to the optical nerve. In the literature, good visual capacities could be preserved in the majority of patients (68%-91%) [3,8,15], but attention should be paid to keratitis, a serious side effect in 24% of patients in the Esmaeli study, which can lead to blindness [10]. Consequently some authors discuss the delineation of detailed intraorbital structures as retina or sclera for toxicity evaluation [6]. It has to be kept in mind that visual impairment or blindness is a real danger of the malignancy itself, about which the patient has to be counseled. Despite that fact, high-grade toxicities (grade III or IV) are rare and may involve skin, mucous membranes, cornea and conjunctiva [8,10,18].
Conclusion
The combination of eye-sparing and therefore, not mutilating, surgery and postoperative-radiotherapy proved to be effective. Ongoing discussions focus on the extent of surgery needed for longterm survival and the dose and fractionation of radiotherapy. This case, in accordance with the literature, suggests a dose of at least 60 Gy.
Considering the ocular side effects, patients have to be counseled thoroughly as an impairment of eyesight might be a possible complication of treatment. Therefore, attempts should be undertaken to reduce toxicity and improve patients’ quality of life.
References
- Mallen-St Clair J, Arshi A, Tajudeen B, Abemayor E, St John M. Epidemiology and treatment of lacrimal gland tumors: a population-based cohort analysis. JAMA Otolaryngol Head Neck Surg. 2014; 140: 1110-1116.
- Roshan V, Pathy S, Mallick S, Chander S, Sen S, Chawla B. Adjuvant Radiotherapy with Three-Dimensional Conformal Radiotherapy of Lacrimal Gland Adenoid Cystic Carcinoma. J Clin Diagn Res. 2015; 9: 05-07.
- Natanegara IAAA, Koornneef L, Veenhof K, Gonzáles Gonzáles D, Boukes RJ. An alternative approach for the management of adenocystic carcinoma of the lacrimal gland. Orbit. 1990; 101-105.
- Kahle, Werner, Frotscher, Michael. Taschenatlas Anatomie in 3 Bänden - Nervensystem und Sinnesorgane, 9th Georg Thieme Verlag, Stuttgart. 2005.
- Edge SB, Compton CC. American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17: 1471-1474.
- Orlandi E, Takanen S, Giandini T, Iannacone E, Fontanella W, Locati L, et al. Postoperative radiotherapy with volumetric modulated arc therapy of lacrimal gland carcinoma: two case reports and literature review. Future Oncol Lond Engl. 2014; 10: 2111-2120.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995; 31: 1341-1346.
- Brada M, Henk JM. Radiotherapy for lacrimal gland tumours. Radiother Oncol J Eur Soc Ther Radiol Oncol. 1987; 9: 175-183.
- Ducrey N, Villemure JG, Jaques B. Cystic adenocarcinomas of the lacrymal gland. Klin Monatsblätter Für Augenheilkd. 2002; 219: 231-234.
- Esmaeli B, Yin VT, Hanna EY, Kies MS, William WN, Bell D, et al. Eye-sparing multidisciplinary approach for the management of lacrimal gland carcinoma. Head Neck. 2016; 38: 1258-1262.
- Manousaridis K, Stropahl G, Hingst V, Guthoff R. Adenoid cystic carcinoma of the lacrimal gland: report of two cases and literature review. Klin Monatsblätter Für Augenheilkd. 2011; 228: 54-56.
- Polito E, Leccisotti A. Epithelial malignancies of the lacrimal gland: survival rates after extensive and conservative therapy. Ann Ophthalmol. 1993; 25: 422-426.
- Sanders JC, Mendenhall WM, Werning JW. Adenoid cystic carcinoma of the lacrimal gland. Am J Otolaryngol. 2016; 37: 144-147.
- Tse DT, Kossler AL, Feuer WJ, Benedetto PW. Long-term outcomes of neoadjuvant intra-arterial cytoreductive chemotherapy for lacrimal gland adenoid cystic carcinoma. Ophthalmology. 2013; 120: 1313-1323.
- Skinner HD, Garden AS, Rosenthal DI, Ang KK, Morrison WH, Esmaeli B, et al. Outcomes of malignant tumors of the lacrimal apparatus: the University of Texas MD Anderson Cancer Center experience. Cancer. 2011; 117: 2801-2810.
- Wright JE, Rose GE, Garner A. Primary malignant neoplasms of the lacrimal gland. Br J Ophthalmol. 1992; 76: 401-407.
- Lin YC, Chen KC, Lin CH, Kuo KT, Ko JY, Hong RL. Clinicopathological features of salivary and non-salivary adenoid cystic carcinomas. Int J Oral Maxillofac Surg. 2012; 41: 354-360.
- Hoppe BS, Wolden SL, Zelefsky MJ, Mechalakos JG, Shah JP, Kraus DH, et al. Postoperative intensity-modulated radiation therapy for cancers of the paranasal sinuses, nasal cavity, and lacrimal glands: technique, early outcomes, and toxicity. Head Neck. 2008; 30: 925-932.