
Case Report
Ann Carcinog. 2017; 2(1): 1010.
The Coexistence of Anaplastic Astrocytomas and Arteriovenous Malformation
Oommen A¹, Govindan J² and Abdul Jalal MJ³*
¹Department of Neurosurgery, VPS Lakeshore Hospital, India
²Department of Pathology, VPS Lakeshore Hospital, India
³Department of Rheumatology and Internal Medicine, VPS Lakeshore Hospital, India
*Corresponding author: Muhammed Jasim Abdul Jalal, Senior Registrar, Department of Rheumatology and Internal Medicine, VPS Lakeshore Hospital, Nettoor .P.O. Maradu, NH 47 – Byepass, Ernakulam, Kerala, India
Received: May 05, 2017; Accepted: June 07, 2017; Published: June 15, 2017
Abstract
Coexistence of astrocytoma with cerebral vascular malformations is unusual, especially if both lesions occur separately. Pre-operative Angiogram will help us to identify any co-existing Arteriovenous Malformation (AVM). This will in turn change the treatment strategy for astrocytoma. Pre-operative embolisation for AVM may be done prior to the glioma excision. There is increased risk of bleeding if AVMs are not pre-operatively diagnosed. AVMs co-existing with astrocytomas are rare. We report a case of anaplastic astrocytoma coexisting with an acquired arteriovenous malformation.
Keywords: Anaplastic astrocytoma; Arteriovenous malformations; Acquired AVM; De novo AVM; VEGF
Introduction
An Arteriovenous Malformation (AVM) is a vascular malformation characterized by arteriovenous shunt through a collection of tortuous vessels without an intervening capillary bed. The most common clinical presentation of AVM is headache and seizures. About 15% can be asymptomatic. More than 50% of AVMs present with intracranial hemorrhages. This contributes to about 2% of all hemorrhagic strokes each year. AVM can be treated either by endovascular embolisation, neurosurgery or radiation therapy [1].
The exact causes of AVMs are still unclear. Apart from congenital AVMs, there are theories suggesting various dynamic developmental processes promoting the existence of acquired AVMs. Vascular Endothelial Growth Factors (VEGF) and Transforming Growth Factors (TGF) play a crucial role in the angiogenesis leading to the development of AVMs, thus challenging the theory of congenital occurrence of all cerebral AVMs.
Anaplastic astrocytomas are WHO grade III gliomas. Anaplastic astrocytoma and glioblastoma are estimated to affect 5-8 people per 100,000 in the general population [2]. Anaplastic astrocytomas are more common in adults than children. In adults, anaplastic astrocytomas commonly develop between 30 to 50 years [2].
Grossly anaplastic astrocytomas are Ill-defined with blurred margins. They may have spongy or gelatinous appearance, with micro cysts and calcifications. They may have a clearer distinction from surrounding brain structures than low grade diffuse astrocytomas. Microscopically, they are similar to grade II tumors but more cellular, more atypia, and mitoses are seen. By definition, vascular proliferation and necrosis are absent.
Coexistence of astrocytoma with cerebral vascular malformations is unusual, especially if both such lesions occur as separate part in the brain [3]. Rare coincidences are reported involving mostly gliomas and meningiomas.
Case Report
A 45-year-old male presented with headache and blurring of vision in the right eye of 2 weeks duration. The patient did not have any episodes of seizures, double vision or loss of consciousness. There was no significant past illness. He was conscious and oriented with a GCS of 15/15 [E4V5M6]. He had nominal aphasia. The pupils were equal and reactive to light. There were no gross motor deficits except for a right sided pronator drift. All the DTRs were normal. The plantar responses were flexor bilaterally. He did not have any cerebellar/meningeal signs. Systemic examinations were normal.
Ophthalmological evaluation showed visual acuity of 6/12 in both eyes. Eye movements were equal in all directions. There was no nystagmus. He had normal color vision. Visual field evaluation showed marked deterioration of peripheral field. Fundus examination was normal under mydriasis with short acting mydriatics.
Imaging
MRI brain Axial T2, FLAIR, SWI, DWI coronal FLAIR and sagittal T1W; dynamic MR perfusion imaging; post contrast axial and coronal T1FS, sag 3D MPRAGE and multivoxel MR spectroscopy were performed.
A large 7.1 x 6.0 x 5.3 cm (AP x Transverse x CC) necrotic, non diffusion restricting, left temporoparietal mass with thick irregular enhancing ring was seen. Dynamic MR Perfusion study showed elevated Cerebral Blood Volume (CBV) in the periphery with relative Cerebral Blood Volume (r CBV) up to 16.3. Multivoxel MR Spectroscopy showed elevated choline and reduced NAA in the solid tumor component. Central necrotic core showed elevated lipid/ lactate peak. There was mild elevation of choline and reduction in NAA in peritumoral white matter. Intra lesional foci of hemorrhage were seen demonstrating susceptibility on SW images in tumor rim (Figure 1).
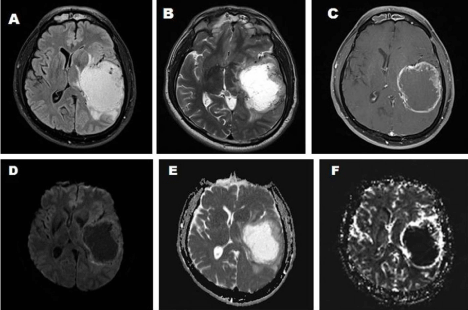
Figure 1: MRI brain showing a large necrotic, non diffusion restricting, left temporoparietal mass with thick irregular enhancing ring, perilesionaloedema and mass
effect.
A. T1 weighted sequence.
B. T2 weighted sequence.
C. T1 Contrast sequence.
D. DWI (Diffusion Weighted Imaging) sequence.
E. DWI – ADC (Apparent Diffusion Coefficient) sequence.
F. Dynamic MR perfusion study showing elevated relative cerebral blood volume.
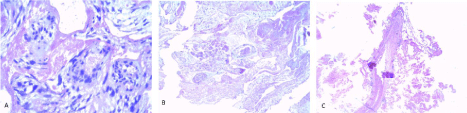
Figure 2: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
There was perilesional edema in the left temporoparietal and peritrigonal deep white matter, posterior limb of left internal capsule and left thalamus. Body and trigone of left ventricle were compressed and effaced. There was also compression and contralateral displacement of left thalamus, third ventricle and midbrain with midline shift by 1.1cm. Ipsilateral ambient cistern was widened. There was mild dilatation of left temporal horn. The imaging features were suggestive of a high grade glioma.
Management
In view of his symptoms, a left temporoparietal craniotomy and near total excision of the space occupying lesion was done under general anaesthesia. Dura was opened. Brain was tense. 60ml of serosanguineous fluid was aspirated using brain canula from the central necrotic material. Brain was lax following aspiration. Near total excision of a highly vascular peripheral growth was done.
Intraoperatively, there was evidence of multiple abnormal small blood vessels throughout the tumor. There was no evidence of any nidus suggestive of AVM. There were no obvious feeding vessels or draining veins. The bleeding stopped after total excision of tumor.
Histopathology
H and E section showed many cavernous vascular spaces with endothelium and dysplastic thick and thin walled vessels containing fibrinous material. Abutting on the walls of these vascular spaces were lobules of a cellular neoplasm, composed of morphologic and GFAP positive, pleomorphic astrocytes, showing mitotic figures and ki67 index of 30-40%. No features of microvascular proliferation and geographic necrosis were seen. VEGF staining showed positivity in the multi-nucleated giant cells in the vessels and macrophages in between the tumor cells. The morphology with Immunohisto Chemistry (IHC) features was compatible with an anaplastic astrocytoma (WHO grade III) with co-existent cavernous/venous type of vascular malformation, probably acquired (Figure 2).
Follow-up
Following the operation, the patient was symptomatically better without any focal neurological deficits.
Discussion
Anaplastic astrocytomas are WHO grade III astrocytic tumors. Their usual site is supratentorial, but can be anywhere in CNS. Preferred sites are frontal and temporal lobes, brain stem, spinal cord. They are uncommon in cerebellum. Neuroimaging shows heterogeneous or patchy enhancement. Grossly anaplastic astrocytomas are Ill-defined with blurred margins. They may have spongy or gelatinous appearance, microcysts, calcification. They may have clearer distinction from surrounding brain structures than low grade diffuse astrocytomas. Microscopically, they are similar to grade II tumors but more cellular, more atypia, and mitoses are seen. By definition, vascular proliferation and necrosis are absent. They have better clinical response to treatment than glioblastoma.
Vascular malformations consisting of abnormal arteries and veins are usually congenital. They can occur at any age, but most often between 20 and 40 years of age. Clinical symptoms of AVM depend on its location. Most frequently, AVM presents with headaches and seizures, but can be asymptomatic as well [15%]. More than 50% of AVMs present with intracranial haemorrhages that account for about 2% of all haemorrhagic strokes each year [4]. The specific treatment for AVM may involve endovascular embolisation, neurosurgery or radiation therapy [5]. The most commonly used grading scale to predict the surgical risk during obliteration is the system described by Spetzler and Martin [6].
The association between vascular malformation and cerebral gliomas is unusual. The lesions, consisting of mixed tumours of glial and vascular origin, particularly of cavernous or arteriovenous type, have been often defined as angiogliomas [7].
The term “angioglioma” is confusing and according to some authors should not be used to determine the true coincidence of vascular and neoplastic glial lesions. Usually, angiogliomas are low grade gliomas and have good prognosis. These are different from the AVMs coexisting with astrocytomas separately. The pathogenetic suggestions consider such angiogliomatic lesions as a result of reactive astroglial neoplastic proliferation secondary to a pre-existing vascular malformation and/or haemorrhages. Microscopically, the hemosiderin-laden macrophages and reactive gliosis could be observed in the vicinity of the lesion. It is important not to confuse such angiogliomatic lesion with high-grade gliomas displaying malignant neovascularity.
The pathogenesis of AVMs remains ill defined. It is a widespread belief that AVMs are congenital [8]. The symptomatic presentation of AVMs in adults before the age of 40, in addition to the de novo AVMs reported in children, support the concept of the temporal vulnerability of vascular elements to a physiologic or environmental trigger [9]. Mechanical, inflammatory, thrombogenic, ischemic/ hypoxic, or hormonal triggers, generally lead to hemodynamic stress [10]. Disturbances of the venous drainage system may contribute to the formation of cerebral AVMs. Venous stenosis, occlusion, or agenesis during embryology or chronic venous hypertension during childhood and adulthood can result in tissue hypoxia and result in the formation of AVM [11]. However, the role of the venous hypertension in the growth of an AVM remains indeterminate. Other vascular lesions such as dural or pialarteriovenous fistula in the brain and spine can develop after trauma, infection, or inflammation. This further support the environmental influences stimulating angiogenesis. Many of these injuries result in the release or increased expression of Transforming Growth Factor (TGF) and Vascular Endothelial Growth Factor (VEGF), which play important roles in angiogenesis. The overstimulation of angiogenesis due to these stressors leads to vascular remodeling and other changes resulting in the maturation of AVMs [12]. Thus AVMs can be congenital or acquired. When acquired, the angiogenesis is predominantly by the growth factors VEGF and TGF.
Up-regulation of VEGF and its receptors has been demonstrated in all gliomas [13]. Astrocytomas are the most common glial tumors. They are subdivided on the basis of how undifferentiated the tissue appears microscopically. Grade I tumors bear the closest resemblance to normal brain. Grade IV are the most undifferentiated. Low grade astrocytomas progress more slowly than their high grade counterparts whose growth can be explosive. Grade IV astrocytoma is also known as glioblastoma, one of the deadliest of all malignant tumors.
While multiple growth factors are potentially operative in glioma angiogenesis, only VEGF is known to induce vascular permeability. Angiogenesis increases markedly from the low grade tumors (grades I and II) to the high grade lesions (grades III and IV). VEGF and its receptors are upregulated in most, but not all, astrocytomas [14]. Low grade astrocytomas which produce VEGF have the same prognosis as high grade lesions [15]. Aberrant VEGF production plays a significant role in the pathophysiology of glioma progression. VEGF is produced in at least four isoforms. Three isoforms, VEGF121 VEGF165 and VEGF189 have been demonstrated in glioblastoma [16].
In 1991, Lombardi et al. did a histological review of 1034 surgically resected AVMs, both angiographically occult and visible [17]. They concluded that oligodendroglioma with AVM-like vasculature appears to be angiographically occult in contrast to AVMs, most which were angiographically obvious and showed arteriovenous shunting. Therefore, histopathologic and angiographic criteria are a must to definitively split apart the coexistence of a true AVM and blood vessel related tumor.
It was in 1965, when Raynor and Kingman described an AVM associated with hemangioblastoma in a 19 year old male [18]. In 1975, Crowell et al. described an angiographically evident AVM in the right temporal lobe of a 17 year old male, which was histopathologically negative [19].
In 1979, Zuccarelloetal described the simultaneous occurrence of malignant astrocytoma (without WHO grade) and an angiographically evident AVM in the left temporal lobe of a 50 year old male [20]. In 1986, Martinez-lage et al. have described a right intraventricular oligodendroglioma co-existing with a right parietal cortical AVM in a 43 years old male who presented with sub arachnoid hemorrhage [21]. The hemorrhage was believed to be caused by the tumor, rather than the AVM.
In 1991, Heffner et al. angiographically documented AVM in the meninges overlying the right frontal benign astrocytoma (WHO grade II) in a 17-year-old male [22].
Furthermore, in 2000 and 2003, the co-existence of AVM and astrocytoma was reinforced by Harris et al. and Kupnicka et al. respectively [23,24]. In 2008, McKinney et al. described an anaplastic oligodendroglioma in a 55 year old female with a previously unremarkable brain imaging [25]. She had a co-existing arteriovenous lesion, which was later on explained as a de-novo occurrence.
AVMs co-existing with gliomas could be identified with preoperative angiogram. These AVMs should undergo pre-operative embolisation prior to tumor excision. In our case, we did not have any suspicion of AVM radiologically and hence pre-operative angiogram was not done.
Conclusion
Anaplastic astrocytomas are highly vascular tumors. These tumors release TGF and VEGF; which can play an important role in angiogenesis and trigger the development of acquired AVMs. Even though acquired AVMs are rare, increasing reports of de novo AVMs challenges the theory of congenital AVM. Pre operative angiogram and embolisation plays an important role in the management of acquired AVMs secondary to vascular tumors.
References
- Laakso A, Dashti R, Juvela S, Niemelä M, Hernesniemi J. Natural history of arteriovenous malformations: presentation, risk of hemorrhage and mortality. Acta Neurochir. 2010; 107: 65-69.
- Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013; 310: 1842-1850.
- Soltanolkotabi M, Schoeneman SE, Dipatri AJ, Hurley MC, Ansari SA, Rajaram V, et al. Juvenile Pilocytic Astrocytoma in Association with Arteriovenous Malformation. Interventional Neuroradiology. 2012; 18: 140-147.
- Naganska E, Matyja E, Pucko E, Zabek M. The coexistence of pleomorphic xanthoastrocytoma and arteriovenous malformation. A case report. Folia Neuropathol. 2013; 51: 269-274.
- Bradac O, Charvat F, Benes V. Treatment for brain arteriovenous malformation in the 1998-2011 period and review of the literature. Acta Neurochir (Wien). 2013; 155: 199-209.
- Rodriguez-Hernandez A, Kim H, Pourmohamad T, Young WL, Lawton MT, University of California SFAMSP. Cerebellar arteriovenous malformations: anatomic subtypes, surgical results, and increased predictive accuracy of the supplementary grading system. Neurosurgery. 2012; 71: 1111-1124.
- Gazzeri R, De Bonis C, Carotenuto V, Catapano D, d’Angelo V, Galarza M. Association between cavernous angioma and cerebral glioma. Report of two cases and literature review of so-called angiogliomas. Neurocirugia (Astur). 2011; 22: 562-566.
- Neil JA, Li D, Stiefel MF, Hu YC. Symptomatic de novo arteriovenous malformation in an adult: Case report and review of the literature. Surgical Neurology International. 2014; 5: 148.
- Stevens J, Leach JL, Abruzzo T, Jones BV. De novo cerebral arteriovenous malformation: Case report and literature review. AJNR Am J Neuroradiol. 2009; 30: 111-112.
- Bulsara KR, Alexander MJ, Villavicencio AT, Graffagnino C. De novo cerebral arteriovenous malformation: Case report. Neurosurgery. 2002; 50: 1137-1140.
- Pietila TA, Zabramski JM, Thellier-Janko A, Duveneck K, Bichard WD, Brock M, et al. Animal model for cerebral arteriovenous malformation. ActaNeurochir (Wien). 2000; 142: 1231-1240.
- Sure U, Butz N, Schlegel J, Siegel AM, Wakat JP, Mennel HD, et al. Endothelial proliferation, neoangiogenesis, and potential de novo generation of cerebrovascular malformations. J Neurosurg. 2001; 94: 972-977.
- Pietsch T, Valter MM, Wolf HK, von Deimling A, Huang HJ, Cavenee WK, et al. Expression and distribution of vascular endothelial growth factor protein in human brain tumors. Acta Neuropathol. 1997; 93: 109-117.
- Berkman RA, Merrill MJ, Reinhold WC, Monacci WT, Saxena A, Clark WC, et al. Expression of the vascular permeability factor/vascular endothelial growth factor gene in central nervous system neoplasms. J Clin Invest. 1993; 91: 153-159.
- Yao Y, Kubota T, Sato K, Kitai R. Takeuchi H. Arishima H. Prognostic value of vascular endothelial growth factor and its receptors flt-1 and flk-1 in astrocytic tumors. Acta Neurochir (Wien). 2001; 143: 159-166.
- Machein MR, Kullmer J, Fiebich BL, Plate KH, Warnke PC. Vascular endothelial growth factor expression, vascular volume, and capillary permeability in human brain tumors. Neurosurgery. 1999; 44: 732-740.
- Lombardi D, Scheithauer BW, Piepgras D, Meyer FB, Forbes GS. “Angioglioma” and the arteriovenous malformation-glioma association. J Neurosurg. 1991; 75: 589-596.
- Raynor RB, Kingman AFJ. Hemangioblastoma and vascular malformations as one lesion. Arch Neurol. 1965; 12: 38-48.
- Crowell RM, DeGirolami U, Sweet WH. Arteriovenous malformation and oligodendroglioma. Case Report. J Neurosurg. 1975; 43: 108-111.
- Zuccarello M, Giordano R, Scanarini M. Malignant astrocytoma associated with arteriovenous malformation. Case Report. Acta Neurochir. 1979; 50: 305-309.
- Martinez-Lage JF, Poza M, Esteban JA, Sola J. Subarachnoid hemorrhage in the presence of a cerebral arteriovenous malformation and an intraventricular oligodendroglioma: case report. Neurosurgery. 1986; 19: 125-128.
- Heffner RR, Porro RS, Deck MDF. Benign astrocytoma associated with arteriovenous malformation. J Neurosurg. 1971; 35: 229-233.
- Harris OA, Chang SD, Harris BT, Adler JR. Acquired cerebral arteriovenous malformation induced by an anaplastic astrocytoma: an interesting case. Neurol Res. 2000; 22: 473-477.
- Kupnicka DJ, Sikorska B, Klimek A, Kordek R, Liberski PP. Angioganglioglioma: A transitional form between angioglioma and ganglioglioma? Ultrastruct Pathol. 2003; 27: 423-432.
- McKinney JS, Steineke T, Nochlin D, Brisman JL. De novo formation of large arteriovenous shunting and a vascular nidus mimicking an arteriovenous malformation within an anaplastic oligodendroglioma: treatment with embolization and resection. J Neurosurg. 2008; 109: 1098-1102.