
Case Report
Ann Carcinog. 2019; 4(1): 1019.
An Unusual Pancreatic Tumor!
Damiri A1,2*, Chahdi H1,2, Setti K1,2, Oukabli M1,2 and Bouzidi AL1,2
¹Department of Pathology, Military Hospital Mohammed V Rabat, Morocco
²Faculty of Medicine and Pharmacy of Rabat (FMPR), Mohamed V Rabat, Morocco
*Corresponding author: Damiri A, Department of Pathology, Military Hospital Mohammed V Rabat, Morocco
Received: October 09, 2019; Accepted: November 09, 2019; Published: November 16, 2019
Abstract
Adenosquamous Carcinoma of the Pancreas (ASCP) is a serious and a rare entity. Like adenocarcinoma of the pancreas, overall survival is poor.
We report a case of a 49-years-old woman, hospitalized for the management of a tumor in the tail of the pancreas. The histological study concluded to adenosquamous carcinoma.
The treatment of this type of tumor is similar to that of acinar adenocarcinoma. Despite the therapeutic progress the prognosis remains dark.
Keywords: Pancreas; Adenosquamous; Carcinoma; Histology
Introduction
Pancreatic cancer is a serious and deadly disease. In 2014, more than 46420 new cases were diagnosed with 39590 deaths, making it the fourth leading cause of cancer deaths in the United States [1]. The most diagnosed histological type is adenocarcinoma [2]. ASCP is a rare entity; its frequency is between 0.38% and 10% of all pancreatic exocrine tumors [2-3]. This tumour was first described in 1907 by Gotthold Herheimer, who called it a carcinoid tumor [4]. Compared to pancreatic adenocarcinoma, overall survival is low at 5 years [5].
Case presentation
A 49 -year-old woman who presented for cholestatic jaundice with symptoms of pruritus, intermittent epigastralgia with discolored stools and dark urine.
ACE and CA 19-9 levels are elevated with anemia and positive cholestasis blood test.
An abdominal MRI was performed revealed a cystic lesion of tail the pancreas measuring 4cm with transverse colon invasion and the stomach (Figure 1A). Abdominal CT showing the presence of multiple hepatic lesions (Figure 1B).
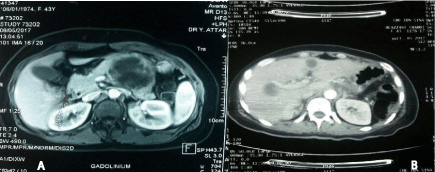
Figure 1: Radiological aspects: A: MRI: solid and cystic formation of tail the
pancreas (Red Arrow) (T1 sequence axial section). B: Abdominal scan: liver
metastases (axial section).
A palliative splenopancreatectomy was performed.
The surgical specimen was sent to the pathology anatomy laboratory. Macroscopic examination shows the presence of a yellowwhite tumor of half-fleshy mid-cystic nature, centered by a friable necrosis measuring 45x35 mm (Figure 2).
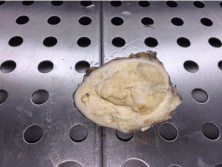
Figure 2: Macroscopic appearance: Solid and cystic mass of tail the
pancreas.
The histological study shows dual-component tumor proliferation: the first adenocarcinomatous essentially constituted of tubes and the second squamous made of cords and cellular clusters (Figure 3). Tumor cells exhibit cyto-nuclear atypias in favor of malignancy with presence of vascular embolus. The Lymph node dissection found 5 lymph nodes metastatic.
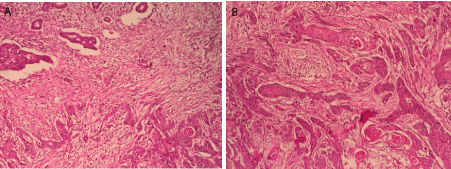
Figure 3: Dual-component tumor proliferation at low magnification HEx100
(A) and at medium magnification HE x200 (B).
Discussion
Although adenosquamous carcinoma is recognized as a rare type of exocrine pancreatic carcinoma, Ishikawa et al. [6] noted that more accurate histopathological examination of the resected specimen may more frequently reveal the existence of the squamous contingent in adenocarcinoma of the pancreas. Yamaguchi et al. [7] also reported that two out of eight cases of adenocarcinoma, has a squamous contingent were revealed by careful examination.
Most patients with this tumour are men in the sixth decade of life, who complain of abdominal pain and weight loss. Serum levels of Carbohydrate Antigen (CA) 19-9 and Carcinoembryonic Antigen (CEA) are largely elevated but not specific. In a series of six patients with adenosquamous carcinoma reported by Kitagawa et al. [8], CA 19-9 was elevated in three patients.
On ultrasound and the scanner, the tumor is often solid. Fujisaki et al. [9] and others [10] reported central necrosis in adenosquamous carcinoma, which is present in our case. Hepatic metastases have also been reported in patients with this type of cancer [11]. This necrosis is secondary to insufficient angiogenesis relative to the growth of the tumor. These findings may suggest that the presence of central necrosis is indicative of adenosquamous carcinoma or squamous cell carcinoma of the pancreas.
In ASCP, the clinical course is less favorable than that of adenocarcinoma. Kitagawa et al. reported that the average survival of six patients with adenosquamous carcinoma of the pancreas was 8.6 months [12].
There is currently no protocol for treating patients with ASCP. According to several studies, surgical treatment is similar to that of acinar adenocarcinoma [13]; it depends on the location of the tumor [13]. Thus, the surgical management remains the best opportunity to achieve a sustainable survival [13].
The role of neoadjuvant and adjuvant chemotherapy is unclear [13]. Some publications have highlighted the efficacy of 5-fluorouracil as a product used in this context [14]. In a retrospective series of 62 patients with ASCP, 14 out of 48 patients received neoadjuvant platinum chemotherapy. Patients who received platinum-based therapy had an overall median survival of 19.1 months instead of 10.7 months for those who did not receive it (P = 0.011) [14]. The place of radiotherapy in addition to surgical resection is unclear [13-16]. In two retrospective studies, the adjuvant radiotherapy did not show any benefit in overall survival for those who received adjuvant therapy compared to those who did not receive it [13]. In patients who did not receive radiation therapy, their 2-year survival rate was 9% and the median survival time was 6 months. This difference in survival is not statistically significant [13].
The role of radiation therapy as an adjunct to resection of ASCP is also unclear [17]. Two retrospective studies examined adjuvant radiation therapy, but did not show a benefit in overall survival for those that received adjuvant therapy vs those who did not. In a previously published literature review of 30 patients who received radiation therapy either intra and/or postoperatively, the 2-year survival rate was 20% and median survival 13 mo [18]. In the patients who did not receive radiation therapy their 2-year survival rate was 9% and median survival period was 6 mo. Despite the differences in survival between the 2 groups, they did not reach statistical significance [18].
Conclusion
ASCP is an aggressive variant of pancreatic carcinoma. Radiology locates the tumor and allows a loco-regional and general extension assessment. Only the histological study makes it possible to pose the exact diagnosis.
The surgical treatment is similar to that of acinar adenocarcinoma. The place of radio-chemotherapy is still debated.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics. CA Cancer J Clin. 2014; 64: 9-29.
- Fitzgerald TL, Hickner ZJ, Schmitz M, Kort EJ. Changing incidence of pancreatic neoplasms: a 16-year review of statewide tumor registry. Pancreas. 2008; 37: 134-138.
- Hsu JT, Yeh CN, Chen YR, Chen HM, Hwang TL, Jan YY, et al. Adenosquamous carcinoma of the pancreas. Digestion. 2005; 72: 104-108.
- Madura JA, Jarman BT, Doherty MG, Yum MN, Howard TJ. Adenosquamous carcinoma of the pancreas. Arch Surg. 1999; 134: 599-603.
- Smoot RL, Zhang L, Sebo TJ, Que FG. Adenosquamous carcinoma of the pancreas: a single-institution experience comparing resection and palliative care. J Am Coll Surg. 2008; 207: 368-370.
- Ishikawa O, Matsui Y, Aoki I, Iwanaga T, Terasawa T, Wada A. Adenosquamous carcinoma of the pancreas. A clinicopathologic study and report of three cases. Cancer. 1980; 46: 1192-1196.
- Yamaguchi K, Enjoji M. Adenosquamous carcinoma of the pancreas. A clinicopathologic study. J Surg Oncol. 1991; 47: 109-116.
- Kitagawa T, Ohta T, Sohma M, et al. Clinical study of six patients with adenosquamous carcinoma of the pancreas. Suizou. 1990; 5: 89-96.
- Fujisaki J, Mine T, Akimoto K, et al. A case of adenosquamous carcinoma of the pancreas with a large cyst. Dig Endosc. 1990; 2: 66-70.
- Nagaiwa J, Ariyama J, Suyama M, et al. Adenosquamous carcinoma of the pancreas. A case report. Fukubugazousindan. 1990; 10: 119-125.
- Hachiya H, Miyaji M, Katagiri K, Hoshino M, Hayakawa T, Kawamura Y, et al. An autopsy case of calcified squamous cell carcinoma of the pancreas with cystic hepatic metastasis. Jpn J Gastroenterol. 1988; 85: 1712-1716.
- Carter D, Eggleston JC. Tumors of the lower respiratory tract. In: Hartmann WH, Cowan WR, eds. Atlas of tumor pathology. Second Series, Fascicle 17. Washington, DC: Armed Forces Institute of Pathology. 1980.
- Okabayashi T, Hanazaki K. Surgical outcome of adenosquamous carcinoma of the pancreas. World J Gastroenterol. 2008; 14: 6765-6770.
- Wild AT, Dholakia AS, Fan KY, Kumar R, Moningi S, Rosati LM, et al. Efficacy of platinum chemotherapy agents in the adjuvant setting for adenosquamous carcinoma of the pancreas. J Gastrointest Oncol. 2015; 6: 115-125.
- Ikeda Y, Ezaki M, Hayashi I, Yasuda D, Nakayama K, Kono A. Establishment and characterization of human pancreatic cancer cell lines in tissue culture and in nude mice. Jpn J Cancer Res. 1990; 81: 987-993.
- Meitner PA, Kajiji SM, LaPosta-Frazier N, Bogaars HA, Jolly GA, Dexter DL, et al. “COLO 357,” a human pancreatic adenosquamous carcinoma: growth in artificial capillary culture and in nude mice. Cancer Res. 1983; 43: 5978- 5985.
- Okabayashi T, Hanazaki K. Surgical outcome of adenosquamous carcinoma of the pancreas. World J Gastroenterol. 2008; 14: 6765-6770.
- Erkut B, Sherri Z, Ron K, Han H, Whatcott CJ, Gatalica Z, et al. Adenosquamous carcinoma of the pancreas: Molecular characterization of 23 patients along with a literature review. World J Gastrointest Oncol. 2015; 7: 132-140.