
Rapid Communication
Ann Carcinog. 2019; 4(1): 1020.
Cytogenetic and Genomic Investigations in River Buffaloes Raised in Farms Located in Urban and Rural Areas of Campania Region (Southern-Italy)
Iannuzzi A1*, Perucatti A1, Genualdo V1, Rossetti C1, Iorio C1, Caputi Jambrenghi A2, Giannico F2, Andreassi MG3 and Iannuzzi L1
¹National Research Council (CNR), ISPAAM, Laboratory of Animal Cytogenetics and Genomics, Naples, Italy
²Department of Animal Production, Agricultural Faculty of Sciences, University of Bari, Bari, Italy
³CNR Institute of Clinical Physiology, Pisa, Italy
*Corresponding author: Iannuzzi A, National Research Council (CNR), ISPAAM, Laboratory of Animal Cytogenetics and Genomics, via Argine 1085, 80147 Naples, Italy
Received: October 29, 2019; Accepted: November 28, 2019; Published: December 05, 2019
Abstract
This study aimed to check possible differences between long- and shortterm DNA damages in lymphocytes of river buffalo cows raised in urban and rural areas by both cytogenetic and genomic tests. Two groups of buffaloes, homogeneous for age, sex and feeding, were studied: group A (Naples district) was raised in an urban area, while group B (Salerno district) was raised in a rural area. Three long-term DNA damage tests (CA, SCE and CBMN) and one short-term DNA damage test (RLTL) have been performed on both groups. Interestingly, no statistical differences were found between the two groups for each test, supporting the possible restarting of normal environmental conditions in the urban area, considered potentially polluted in the past.
Keywords: River Buffalo; Cytogenetic Tests; Genomic Test; Environment; Pollution
Abbreviations
CA: Chromatid and Chromosome Breaks; SCE: Sister Chromatid Exchange; CBMN: Cytokinesis-Block Micronucleus; RLTL: Relative Leukocyte Telomere Length; CCL: Concentration of Contamination Level; BCI: Binucleated Cell Index; MN: Micronuclei, BN: Binucleated; MMQPCR: Monochrome Multiplex Quantitative PCR; STL: Sperm Telomere Length; SCG: Single Copy Gene; NTC: No Template Control
Introduction
Several pollutant types, partially volatile and derived from human activities, even if present at low concentration in the environment, can interfere with physiological systems and therefore the capacity of ruminants and other animals to reproduce, rear offspring or fight disease. Indeed, the exposition to exogenous agents, alone or in combination, can lead to a variety of modifications on DNA composition, resulting in genome and chromosome alterations. Chromosomes are still considered one of the best biological markers to monitor damages associated with natural or in vitro exposure to environmental mutagens. Recently, in addition to routine cytogenetic tests such as CA, SCE and CBMN [1-3], also a genomic test has been used. The analysis of the RLTL (expressed as telomere length relative to a single copy reference gene) has been performed to check DNA-damages in human populations exposed to pollutants in both leucocytes [4] and sperms [5]. For this reason, the monitoring of livestock population by cyto-genomic tests might represent a good tool to indirectly control of the food chain, to preserve health problems, and to avoid management and income issues at the farm level.
Several studies have investigated the mortality rates in the polluted areas of the Campania region highlighting an increased level of mortality in the human population of Naples and Caserta districts, compared to the remaining ones (Avellino, Benevento, and Salerno) [6]. However, the CCL analyses, recently performed by the official Environmental Regional Agency of Campania region (ARPAC http:// www.arpacampania.it), within different areas of Naples and Caserta districts, reported that only the 6.2% of the areas earlier retained polluted are now forbidden for agro-food production [7].
The study is also a comparison between long- (cytogenetic) and short- (genomic) term tests to be applied for environmental assays. In our knowledge, this is the first time that this type of study has been performed in domestic animals.
Materials and Methods
Two groups of Italian Mediterranean river buffaloes (20 animals per group), homogeneous for age (2-3 years old) and sex (females), randomly chosen from two farms have been selected for the analysis. The two farms used also similar diet and vitamin supplementation and were located in two different areas of the Campania region: the group A in a urban area of Naples district with high environmental pressure in the past, and the group B located in an rural area at low environmental pressure (Salerno district), considered as control (Figure 1). The study was also approved by the Ethical Commission of the National Research Council (CNR), ISPAAM of Naples (reg. n 653 of June 5, 2017).
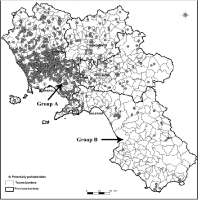
Figure 1: Geographic representation of Campania region (southern-Italy)
with the indication of areas earlier retained potentially polluted by the
official ARPAC agency (http://www.sito.regione.campania.it/burc/pdf05/
burcsp09_09_05/pianoregionale_bonifica.pdf). Arrows indicate the locations
of the two farms used for the study: one in a urban area (Group A) and the
other one (Group B) in a rural area.
We have performed three cytogenetic (CA, SCE and CBMN) and one genomic (RLTL) tests. The CA and SCE tests have required peripheral blood cultures, hypotonic treatment, fixations and acridine orange stain [8]. 100 and 35 cells (with entire chromosome set, 2n=50) for each animal, were studied for CA- and SCE-tests, respectively [9]. For CA-test, only no linear breaks were considered (Figure 2). The CBMN test has required different blood cultures, hypotonic treatment, fixations and Giemsa stain [10]. The nuclear division index and the percentage of BCI were examined for each sample. The analysis of MN was performed on 1000 BN cells, for each sample, with preserved cytoplasm (Figure 2). The RLTL test was performed by MMQPCR according to the method described by Cawthon et al. [11] (Figure 3). The RLTL was measured as T/S ratio (average ratio of telomere repeat copy number to a scg) for each sample and calculated using the ΔΔCt method: T/S ratio= 2 - (ΔCttelomereΔCtscg) = 2ΔΔCt [12].
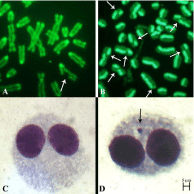
Figure 2: Details of CA (A), SCE (B), BC (C) and MN (D) in river buffalo
lymphocytes.
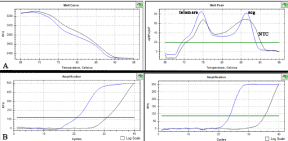
Figure 3: (A) Representative dissociation curve (blue) for telomere and SCG
using CFX RT-PCR System. The 74°C read corresponds to the Ct value for
the telomere amplification while the 87°C read corresponds to the Ct (cycle
threshold) value for the scg amplification. (B) Representative amplification Ct
curve for telomere (left) and scg (right). NTC is also reported (black).
For each animal and animal group, it has been estimated the mean values and the standard deviation. The mean value of each test was compared by the t-student test, and Bonferroni correction was applied as a default restriction. Differences were considered significant for P ‹ 0.05.
Results
Data obtained for the two groups of buffaloes are summarized in Table 1. No significant differences were found between the two examined groups of buffaloes comparing the results obtained by four different tests, of which three at long- (CA, SCE and CBMN) and one (RLTL) at short-term of DNA damage.
PARAMETERS
Group A
Group B
(p-Value)
CA
0.06±0.26
0.07±0.27
0.48
SCE
8.96±3.86
9.18±4.35
0.32
CBMN BCI
76.70±7.32
74.89±6.32
0.75
MN
1.52±1.87
1.76±2.07
0.73
T/S ratio
1.34±0.92
1.44±1.21
0.78
a, b Values within columns with different superscripts are significantly different; P< 0.05.
Table 1: Mean values of CA, SCE, CBMN (BCI and MN) and RLTL (T/S ratio) in the two groups of river buffalo cows reared in farms from urban (A) and rural (B) areas of Campania Region.
Discussion
It is interesting to note that all four tests gave similar results between the two groups (Figure 4), suggesting that the RLTL test could be very useful for environmental analyses, being faster than other tests and no requiring cell cultures but only DNA-extraction and RT-PCR analyses. Our results agree also with recent studies performed in human males living in areas of the Campania region at high and low environmental pressure [13]. Indeed, these authors did not identify statistical differences when analysing RLTL, but they found statistical differences when examined for STL. The authors could not explain these data, probably due to a large variability of age of male samples (18-35 years), the low number of subjects and RLTL age-dependent attrition in comparison to STL, longer in older human sperm donors than in younger ones [14]. The two groups of buffaloes investigated in our study showed no sensitivity to the different environmental pressure. We could hypothesize that our data are likely to represent also those in other areas of Naples and Caserta districts, earlier reported with a high environmental pressure (Figure 1). Indeed, the most recent data published by the ARPAC on the CCL over the permitted value, with at least one contaminant and performed upon 145 hectares of soil in areas of Naples and Caserta provinces earlier found polluted, 101 hectares (67.4%) were found suitable for the agro-food production and only 9 hectares (6.2%) resulted forbidden for both agri-food and pasture. The remaining 35.2 hectares have been classified suitable for agriculture use with some limits for some agro-food production in specific conditions [7]. Furthermore, the Napoli district area, considered in this experiment, has been classified as a low-environmental impact area in 2014.
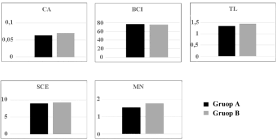
Figure 4: Graphic representation of the two river buffalo groups reared in
farms from urban (A) and rural (B) areas of the Campania region.
Conclusion
Further cyto-genomic analyses, on both leucocytes and sperms, should be performed in larger samples (and areas) of both animals and humans living in different environmental conditions to fully confirm the environmental improvements that occurred in these two districts covering a large area hosting about 3 millions of people. RLTL-test could be largely applied on both humans and animals exposed to environmental pressures.
Conflict of Interest Statement
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Acknowledgments
The authors wish to thank Mr. Domenico Incarnato (CNRISPAAM of Naples), for excellent technical support. The study has been partially supported by the project, Genobu PON1_486.
References
- Perucatti A, Di Meo GP, Albarella S, Ciotola F, Incarnato D, Jambrenghi AC, et al. Increased frequencies of both chromosome abnormalities and SCEs in two sheep flocks exposed to high dioxin levels during pasturage. Mutagenesis. 2006; 21: 67-75.
- Genualdo V, Perucatti A, Iannuzzi A, Di Meo GP, Spagnuolo SM, Caputi- Jambrenghi A, et al. Chromosome fragility in river buffalo cows exposed to dioxins. Journal of Applied Genetics. 2012; 53: 221-226.
- Iannuzzi A, Genualdo V, Perucatti A, Rossetti C, Incarnato D, Caputi- Jambrenghi A, et al. Cytogenetic and genomic assays in river buffalo (Bubalus bubalis, 2n=50) cows raised in urban and rural areas. 12th European Cytogenomics Conference 2019, SALZBURG (AT). 2019; 12: 30.
- Shin JY, Choi YY, Jeon HS, Hwang JH, Kim SA, Kang JH, et al. Lowdose persistent organic pollutants increased telomere length in peripheral leukocytes of healthy Koreans. Mutagenesis. 2010; 25: 511-516.
- Rocca MS, Speltra E, Menegazzo M, Garolla A, Foresta C, Ferlin A. Sperm telomere length as a parameter of sperm quality in normozoospermic men. Human Reproduction. 2016; 31: 1158-1163.
- Mazza A, Piscitelli P, Falco A, Santoro ML, Colangelo M, Imbriani G, et al. Heavy Environmental Pressure in Campania and Other Italian Regions: A Short Review of Available Evidence. Int J Environ Res Public Health. 2018; 15.
- Marro C, Iorio R, Bardari R. “Terra dei fuochi”: i risultati delle ultime analisi. ARPA CAMPANIA AMBIENTE. 2017; 6-7.
- Perucatti A, Genualdo V, Pauciullo A, Iorio C, Incarnato D, Rossetti C, et al. Cytogenetic tests reveal no toxicity in lymphocytes of rabbit (Oryctolagus cuniculus, 2n=44) feed in presence of verbascoside and/or lycopene. Food and Chemical Toxicology. 2018; 114: 311-315.
- Iannuzzi A, Perucatti A, Genualdo V, Pauciullo A, Melis R, Porqueddu C, et al. Sister chromatid exchange test in river buffalo lymphocytes treated in vitro with furocoumarin extracts. Mutagenesis. 2016; 31: 547-51.
- Sannino A, Zeni O, Massa R, Gialanella G, Grossi G, et al. Adaptive response in human blood lymphocytes exposed to non-ionizing radiofrequency fields: resistance to ionizing radiation-induced damage. J Radiat Res. 2014; 55: 210-217.
- Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009; 37: e21.
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001; 29: e45.
- Vecoli C, Montano L, Borghini A, Notari T, Guglielmino A, Mercuri A, et al. Effects of Highly Polluted Environment on Sperm Telomere Length: A Pilot Study. Int J Mol Sci. 2017; 18.
- Kimura M, Stone RC, Hunt SC, Skurnick J, Lu X, Cao X, et al. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nature Protocols. 2010; 5: 1596-1607.