
Special Article – Mitral Valve
Austin Cardio & Cardiovasc Case Rep. 2016; 1(3): 1011.
Delayed Spleen Rupture after Post Endocarditis Mitral Minimally Invasive Surgery: An Unexpected Finding
Chirichilli I¹, Iaccarino A², D’Ascoli R³, Rose D4, Saade W², Frati G5 and Greco E²*
¹Cardiac Surgery Department, European Hospital, Italy
²Department of Cardiovascular, Respiratory, Nephrological, Anesthesiological, and Geriatric Sciences, Policlinico Umberto I - Sapienza University of Rome, Italy
³Department of Cardiac Surgery, Ospedale dell’Angelo, Mestre, Italy
4Department of Cardiac Surgery, St Thomas Hospital, Westmister Bridge Road, London, UK
5Department of Medico-Surgical Sciences and Biotechnologies, Sapienza University of Rome, Rome, Italy
*Corresponding author: Greco E, Department of Cardiovascular, Repiratory, Nephrological Anesthesiological and Geriatric Sciences, Policlinico Umberto I – Sapienza University of Rome, Italy
Received: July 27, 2016; Accepted: October 19, 2016; Published: October 21, 2016
Abstract
We present a case of a delayed spontaneous spleen rupture after a poststaphylococcal endocarditis treated with Port-access and Video assisted mitral valve replacement and complicated with an undiagnosed splenic abscess. This case suggests that patients with infective endocarditis should be routinely evaluated for splenic lesions with routine abdominal CT scanning to rule out the presence of splenic abscess or to characterize the size, the location and the evolution of splenic infarcts. Life-threatening sudden complications can be related to undiagnosed splenic abscess after post endocarditis valve surgery.
Keywords: Splenic abscess; Endocarditis; CT-scan; Port-access mitral surgery
Introduction
Infective Endocarditis (IE) is a deadly disease and despite the improvements in its management, it remains associated with high mortality and severe complications. Patients with the highest risk of infective endocarditis can be placed in three categories:
(1) Patients with a prosthetic valve or transcatheter-implanted prostheses and homografts and patients with prosthetic material used for cardiac valve repair.
(2) Patients with previous diagnosis of infective endocarditis.
(3) Patients with untreated cyanotic congenital heart disease or patients who have postoperative palliative shunts, conduits or other prostheses [1].
Embolic events are a frequent and life-threatening complication of IE, related to the migration of cardiac vegetations. The brain and spleen are the most frequent sites of embolism in left-sided IE, while pulmonary embolism is frequent in native right-sided and pacemaker lead IE. Splenic rupture is usually a surgical complication of splenic infarction. There are numerous etiologies of splenic rupture: trauma, malignant or benign hematologic disorders, embolic disorders, vascular disorders, or autoimmune/collagen vascular disease [2,3]. Spontaneous spleen rupture caused by splenic infarction due to embolism, firstly reported in 1919 by Lake et al. [4], is an extremely rare but life-threatening complication of cardiac valve endocarditis. Here we describe a case of mitral valve replacement for infective endocarditis complicated by hemoperitoneum due to spontaneous splenic abscess rupture two weeks after surgery.
Case Presentation
A 60-year-old man was admitted to our Institution to undergo a mitral valve surgery for severe regurgitation due to anterior leaflet prolapse. The anamnestic collection revealed a previous history of urologic procedure consisting of ureteral reimplantation. He denied any intravenous drug abuse. A few months later due to a sudden dispnoea, he was admitted to the Hospital. Physical examination revealed a grade III/IV Levine systolic ejection murmur on the fourth left sternal border. The liver and the spleen were not palpable. The white blood cell count was 6570/mm³ and C-Reactive Protein was 2000μg/L. A transthoracic echocardiography revealed severe mitral regurgitation due to flail of anterior leaflet in A2 scallop. Left ventricular size and function were within the normal ranges. A Portaccess and Video assisted mitral valve surgery was scheduled using Endo-cardiopulmonary bypass and endo-aortic occlusion. The surgical technique has been previously described [5-12]. A left-atrial approach to the mitral valve was accomplished and the valve was exposed. It was totally destroyed, with flail and tears of the anterior leaflet, a large amount of soft material in both side of the leaflet, showing a subacute endocarditis pattern (Figure 1).
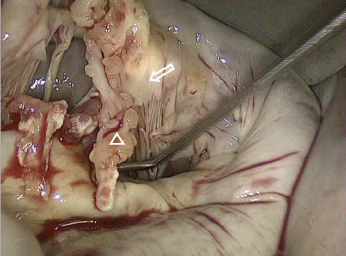
Figure 1: Intra-operative picture showing a subacute endocarditis pattern
with flail and tears of the anterior leaflet (white arrow) and a large amount of
soft material (white triangle) in both side of the leaflet.
The native valve, which was excised, resulted positive for Staphylococcus Aureus. A valve replacement was performed using a biological prosthesis (Carpentier Edwards n.31). Total endoclamping time was 65 minutes and total cardio-pulmonary bypass time was 100 minutes. The patient went off bypass without any concern. Post-bypass transesophageal echocardiogram revealed a normal function of the mitral prosthesis with no evidence of any paravalvular leakage. The immediate postoperative course was uneventful for the first two weeks with no signs of relapsing endocarditis and the patient was discharged to rehabilitation ward. Serial postoperative microbiological blood cultures were sterile with normal inflammatory markers and blood tests except for a mildly low platelet count. No fever was reported. On day 15 after the intervention of valve replacement, during his rehabilitative course, he complained of sudden dull pain on his left upper abdomen and suddenly fell into shock. An emergency abdominal Computed Tomography-scan revealed a massive hemoperitoneum and suspicion of rupture of spleen abscess (Figure 2).
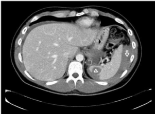
Figure 2: The emergency abdominal CT-scan revealing a massive
hemoperitoneum (white arrow) due to sudden rupture of spleen abscess
(white triangle).
An urgent laparotomy was performed; it revealed a huge amount of intra-abdominal blood wholly surrounding the spleen certainly derived from a ruptured splenic subcapsular abscess and subsequently a total splenectomy was accomplished. Intravenous targeted antibiotic therapy was started at the time of admission to Intensive Care Unit and continued for four weeks after splenectomy. The patient was discharged uneventfully on day 15 after laparotomy.
Discussion
Complications of IE may involve cardiac or extra-cardiac structures especially when a septic embolism occurs. The frequency of specific complications depends on several variables; for example the presence of Staphylococcus Aureus infection, diabetes, acute renal insufficiency, vegetation size >15mm or signs of persistent infection could be associated with the development of Septic Shock [1,13,14]. Despite continued progress in the diagnosis and treatment of this condition, IE continues to have an in-hospital mortality of approximately 20%, essentially unchanged over the past 2 decades [15,16]. Spontaneous spleen rupture secondary to abscess is an extremely rare complication of infective endocarditis. An incidence of splenic infarction and abscess of 19% has been reported in valve replacement for “left side” infective endocarditis [17,18]. There are three pathophysiological mechanisms of splenic rupture in endocarditis: 1) rupture of splenic abscess, 2) rupture of a mycotic aneurysm, 3) rupture of a haematoma secondary to suppurating intrasplenic vessel, subcapsular dissection and delayed capsular tear. Although there are no definitive guidelines on the indications for elective splenectomy once multiple splenic infarctions or abscess secondary to endocarditis are diagnosed, it is generally accepted that asymptomatic splenic infarcts, small or in the mid portion of the spleen, do not require any specific surgical therapy. If the splenic infarcts evolves into an abscess, splenectomy should be performed due to the high risk of splenic rupture. Computed tomography is the most sensitive and specific imaging technique to diagnose splenic abscess and should be taken into consideration as a routinary examination in case of infective endocarditis. The typical feature of a splenic abscess on CT scan is a focal lesion of low attenuation with peripheral enhancement following intravenous contrast injection. On the contrary, splenic infarctions generally appear well-defined, peripheral, like wedge-shaped lesions with the apex toward the hilum and the base extending toward the capsule.
In case of large and peripherally located splenic infarct, it is necessary an observational period with serialized CT- scan even if the patient remains completely asymptomatic [19]. From a clinical point of view, it is difficult to distinguish splenic abscess from infarctions as they share similar symptoms and signs. Indeed, in patients with infective endocarditis, splenic abscess or infarctions may encompass the same disease entity evolving in different stages due to septic emboli of the spleen [20]. Consequently, given the huge variety of clinical manifestations of splenic abscess, a high index of suspicion might be mandatory to make the diagnosis in a patient with infective endocarditis.
In conclusion we believe that patients with infective endocarditis should be evaluated for splenic lesions with routine abdominal CT scanning. The presence of a splenic abscess mandates splenectomy.
References
- Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis. European Heart Journal. 2015; 36: 3075-3123.
- Stollberger C, Finsterer J, Pratter A, Kopsa W, Preiser J, Valentin A. Ischemic Stroke and Splenic Rupture in a Case of Streptococcus bovis Endocarditis. Journal of Clinical Microbiology. 2003; 41: 2654-2658.
- Corbi P, Manic H, Donal E, Roblot F, Richer JP, Coisne D, et al. Mycotic aneurysm of the splenic artery. A rare complication of surgically treated infectious endocarditis at the same time as the causal cardiac lesion. Arch. Mal. Coeur Vaiss. 1999; 92: 1221-1224.
- Lake NC, Kevin HK, Irel S. Three uncommon abdominal cases illustrating some pitfalls. Lancet. 1919; 2: 13.
- Greco E, Barriuso C, Castro MA, Fita G, Pomar JL. Port-Access cardiac surgery: from a learning process to the standard. Heart Surg Forum. 2002; 5: 145-149.
- Chirichilli I, D’Ascoli R, Rose D, Frati G, Greco E. Port Access(Thru- PortSystem)video-assisted mitral valve surgery. Journal Of Thoracic Disease. 2013; 5: 680-685.
- Greco E, Zaballos JM, Alvarez L, Urso S, Pulitani I, Sàdaba R, et al. Videoassisted mitral surgery through a micro-access: a safe and reliable reality in the current era. J Heart Valve Dis. 2008; 17: 48-53.
- Greco E, Mestres CA, Cartana R, Pomar JL. Videoassisted cardioscopy for removal of primary left ventricular myxoma. European Journal of Cardio- Thoracic Surgery. 1999; 16: 677-678.
- Casselmann F, Aramendi J, Bentala M, Candolfi P, Coppoolse R, Gersak B, et al. Endoaortic Clamping does not increase the risk of stroke in Minimal Access Mitral Valve Surgery: A Multcenter Experience. Ann Thorac Surg. 2015; 100: 1334-1339.
- Speziale G, Nasso G, Esposito G, Conte M, Greco E et al. Results of mitral valvere repair for Barlow disease (bileaflet prolapse) via right minithoracotomy versus conventional median sternotomy: a randomized trial. J Thorac Cardiovasc Surg. 2011; 142: 77-83.
- Misfeld M, Borger M, Byrne JG, Chitwood WR, Chon L, Galloway A, et al. Cross – sectional survey on minimally invasive mitral valve surgery. Ann Cardiothorac Surg. 2013; 2: 733-738.
- Ruvolo G, Speziale G, Greco E, Mercogliano D, Marino B. “Mold – like) calcification of the left atrium and of the pulmonary vein. Total endoatriectomy in a patient undergoing mitral valve replacement. Eur J Cardiothorac Surg. 1994; 8: 54-55.
- Olmos C, Villacosta I, Fernandez C, Lopez J, Sarria C, Ferrera C, et al. Contemporary epidemiology and prognosis of septic shock in infective endocarditis. Eur Heart J. 2012.
- Galvez-Acebal J, Rodriguez-Banol J, Martinez-Marcos FJ, Reguera JM, Plata A, Ruiz J. Prognostic factors in left-sided endocarditis: results from the Andalusian Multicenter Cohort. BMC Infect Dis. 2010; 10: 17.
- Hasbun R, Vikram HR, Barakat LA, Buonconsejo J, Quagliarello VJ. Complicated left – sided native valve endocarditis in adults. Risk classification for mortality. JAMA. 2003; 289: 1933-1940.
- Wang A. The changing epidemiology in infective endocarditis: the paradox of prophylaxis in the current and future eras. Journal of the American College of Cardiology. 2012; 59: 1977-1978.
- Mei-Hui Yang, Chia-Chan Wu, Wei-Horng Jean, Cheng-Wei Lu,Yueh-Hsun Chuang, Tzu-Yu Lin. Spleen rupture afetr mitral valve replacement for infective endocarditis. Acta Anaesthesiol Taiwan. 2008; 46: 191-193.
- Murdoch DR, Corey GR, Hoen B, Miro´ JM, Fowler VG Jr, Bayer AS, et al. International Collaboration on Endocarditis-Prospective Cohort Study (ICEPCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century. Arch Intern Med. 2009; 169: 463-473.
- Georgios Dimitrakakis, Ulrich Von Oppell, Georgios Zilidis, Anurag Srivastava. Splenic rupture complicating aortic valve replacement for bacterial endocarditis. Interact CardioVasc Thorac Surg. 2008; 7: 138-140.
- Chia-Chi W, Chih Hsin L, Cheng-Yi C, Hung-Wei C. Splenic infarction and abscess complicating infective endocarditis. Am J Emerg Med. 2009; 27: 3-5.