Case Report
Austin J Clin Cardiolog. 2014;1(3): 1020.
Bentall Surgery in a Patient after Aortic Valve Replacement Due to Takayasu Arteritis
Satoshi Matsushita1*, Taira Yamamoto1, Shizuyuki Dohi1, Naoto Tamura2, Yoshinari Takasaki2 and Atsushi Amano1
1Department of Cardiovascular Surgery, Juntendo University, Japan
2Division of Rheumatology, Department of Internal Medicine, Juntendo University, Japan
*Corresponding author: Satoshi Matsushita, Department of Cardiovascular Surgery, Juntendo University, Japan
Received: March 24, 2014; Accepted: April 21, 2014; Published: April 24, 2014
Case
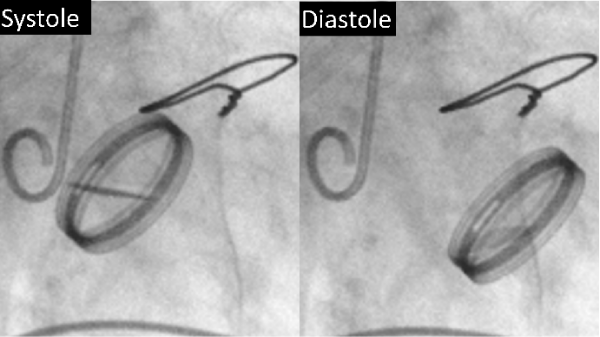
A 57–years old woman underwent an aortic valve replacement (ATS 23–mm mechanical valve, Medtronic ATS Medical, Minneapolis, USA) for the treatment of aortic regurgitation. She was diagnosed with Takayasu arteritis post–operative pathology and prescribed 5 mg prednisolone per day after surgery. At 61 years of age, she required implantation of a ventricular pacemaker device for the treatment of an atrio ventricular block. At 64 years of age, she started experiencing dyspnea on effort. Echocardiography and contrast radiography revealed severe aortic regurgitation due to a flailed mechanical valve (Figure 1) with aortic root ectasia (annulus, 30 mm; Valsalva sinus, 62 mm; and ascending aorta, 43 mm). She underwent Bentall surgery via reopening of the mid–sternotomy.
Figure 1: Preoperative radiographic images of the valve during systole (left) and diastole (right) showing that the replaced valve had significantly moved, indicating detachment–induced valve failure.
Surgical findings
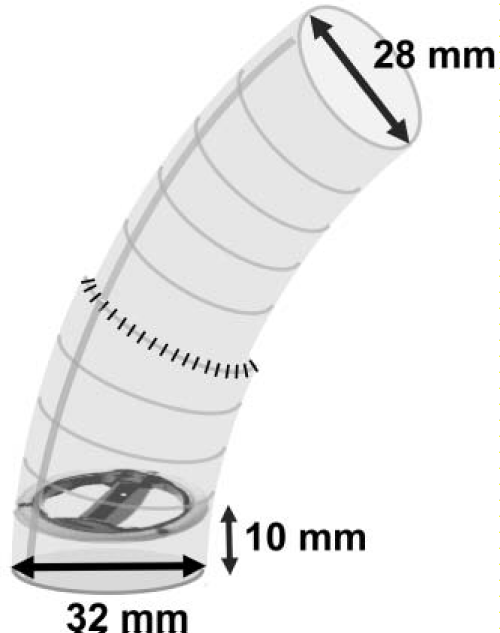
On opening the aortic root, we found that the replaced mechanical valve had flailed and dropped inside the left ventricle. The valve was removed and the new mechanical valve (ATS 29–mm) with a composite graft of woven Dacron (Hemashield 32–mm; Hemashield Technology, Japan) was placed. To create the composite graft, the mechanical valve was sewn with a 10–mm margin from itsproximal end (Figure 2). A coronary button was reconstructed by using the Carrel patch method. For the distal side, another 28–mmDacron graft was connected and the ascending aorta was replaced right before the brachiocephalic artery. Teflon felt was wrappedaround the anastomosis between the graft and native vessel. Afterthe aorta was declamped, mitral regurgitation was observed through transesophageal echocardiography, so a mitral valve replacement (ATS 25–mm) was also performed. The total cross–clamping time was 307 minutes, pump time was 347 minutes, and duration of circulatory arrest was 23 minutes. Postoperatively, she was prescribed 0 mg of prednisolone from the day of the surgery that was reduced to 15 mg upon hospital discharge. The steroid therapy has been continuously managed in collaboration with our hospital’s Division of Rheumatology. The patient is now in her fifth year after the second surgery, and no signs of complications have been seen to date.
Figure 2: The composite graft was designed to suture the mechanical valve with a 10–mm margin from the end of the proximal side. The distal side of the graft was connected to another one (32–28 mm) to adjust the diameter of the native aorta.Discussion
In Takayasu arteritis, which is more common in Asian people, inflammation spreads widely to the aorta and its branches, although they appear to be normal. With long–term steroid administration, in addition to increased inflammation induced by surgical stress, postsurgical valve failure or aneurysm formation has been of concern [1,2]. In this case, the flailing occurred 7 years after the first aortic valve replacement. Since the tissue was assumed to be fragile due to inflammation, we attempted to remove as much of the diseased aortic tissue around the anastomosis by using the skirt implantation technique [3]. Anastomosis of the proximal site was performed inside the left ventricle with interrupted suturing. This might cause intra operative mitral regurgitation; however, a sturdy anastomosisshould be preferred to prevent recurrent surgery. Importantly, antiinflammation therapy, including steroid administration, should be treated in collaboration with an expert in autoimmune diseases in the perioperative period to control the inflammation which is reportedly another key factor for preventing disease progression [4].
References
- Miyata T, Sato O, Deguchi J, Kimura H, Namba T, Kondo K, et al. Anastomotic aneurysms after surgical treatment of Takayasu’s arteritis a 40-year experience. J Vasc Surg. 1998; 27: 438-451.
- Watanabe Y, Matsushita S, Okawa S, Yamabuki K, Gomi S, Hiyama T. Aortic root replacement for prosthetic aortic valve detachment without regurgitation and with enlarged Valsalva’s sinuses and complete atrioventricular block caused by Takayasu’s aortitis. Jpn J Thorac Cardiovasc Surg. 2003; 51: 201-204.
- Moro H, Hayashi J, Okazaki H, Takahashi Y, Eguchi S, Yazawa M, et al. Open heart surgery in patients with systemic diseases requiring steroid treatment. Nihon Kyobu Geka Gakkai Zasshi. 1996; 44: 493-498.
- Chau EM, Cheung KL, Fu KH, Lee JW. Recurrent aortic valve prosthesis dehiscence secondary to aortoarteritis: three case reports and literature review. Int J Cardiol. 1996; 56: 113-118.