Abstract
The present study aimed to compare the hydrogen and methane productions by microbial consortium designated MC1 (Clostridium, Bacillus, Bacteroides and Paenibacillus genus) and MC2 (Clostridium, Raoultella, Klebsiella and Desulfovibrio genus). Both tests used hydrothermally pretreated Sugarcane Bagasse (SCB) as substrate in mesophilic conditions (37°C). The maximum hydrogen productions were 5.33mmol/L for MC1 and 2.45mmol/L for MC2. Methane was produced only by the MC2, reaching 53.65mmol/L. Thus, the culture of MC1 can be used as a source of fermentative hydrogen producer while the MC2 can be a promising source of methanogenic microorganisms which can improve the biogas production.
Keywords: Bioconversion; Hydrogen; Methane; Hydrothermal pretreatment; Cellulolytic substrate
Introduction
The hyper-consumption of non-renewable resources, mainly fossil fuels, has resulted in unprecedented levels of greenhouse gas emissions which are related to carbon dioxide emissions, considered the major cause of current global warming and climate change [1]. Therefore, research and development on biological hydrogen production using microorganisms has advanced, which may relieve the pressure caused by carbon dioxide emissions and the depletion of fossil fuel resources [2].
Hydrogen must be generated from renewable raw materials and used as renewable source of energy. Agricultural waste is a promising alternative biomass to renewable energy production once different kind can be utilized as feedstock for the biological ethanol, hydrogen and biogas productions [3,4]. The relative abundance, the world-wide distribution of these cellulosic materials is attractive factors for generating biotechnological products. The bioconversion of lignocellulosic compounds into hydrogen and biogas can occur at ambient temperature and atmospheric pressure, which is other attractive condition to biohydrogen production [5].
Sugarcane Bagasse (SCB) is a residual product of sugarcane processing, which is one of the most important process adopted in Brazil for fuel production. This residue is used as animal feed or burned to energy recovery. Nevertheless, it could be used as substrate for second generation bioethanol, methane [3] and hydrogen productions [6]. The bioproduction of hydrogen and biogas is recognized as a very promising, environmental friendly and feasible strategy [5], although some factors can affect its effectiveness.Among the factors that affect the bioconversion of lignocellulosic wastes into bioenergy, the high lignin content and cellulose crystallinity have been considered the main cause of low digestibility of the substrate. The SCB is basically composed of cellulose (40-45%), hemicellulose (30-55%) and lignin (20-30%) [3]. In this context, pretreatments have been applied to disrupt the biomass components (cellulose and lignin) and improve the enzymatic digestibility. So, the applicability of pretreatments at industrial scale should be considered i.e., economic viability, minimum generation of microbial inhibitory compounds and fewer environmental impacts [7]. Hydrothermal pretreatment is a process applied to liberate sugars from lignocellulosic materials releasing two fractions, a solid fraction, mainly containing cellulose and lignin, and a liquid fraction (hydrolyzed) containing pentose and hexose. This process can be performed without the addition of chemicals, making it a potential solution for the pretreatment of large quantities of lignocellulosic substrates [8].
The origin of inoculum is also an important factor that affects the biogas and hydrogen production from lignocellulosic biomass, because a small microbial variety can produce cellulolytic enzymes responsible for efficient degradation of the crystalline cellulose structure. Moreover, microbial consortium can work synergistically to produce all enzymes needed for complete cellulose bioconversion [9]. In addition, the use of microbial consortium makes the process simpler, from the point of view of operation and control [10], it is a more robust alternative. The great advantage of the microbial consortium application is regarding the ability to convert many substrates due to its metabolic flexibility, when compared to the pure cultures [11]. Therefore, this study evaluated the effect of inoculum origin on the hydrogen and biogas production from hydrothermally pretreated Sugarcane Bagasse (SCB).
Materials and Methods
Raw materials sugarcane bagasse
The sugarcane bagasse used in this study was provided by São Martinho sugarcane mill (Pradópolis, SP, Brazil).
Pretreatment of sugarcane bagasse
The hydrothermal pretreatment of SCB was carried out in a stainless steel-reactor. The reactor was previously filled with 100mL of water, and then, 5.0g of the substrate was introduced. After substrate addition, the reactor was turned on and set to operate at 200°C with a pressure of 15bar. After 10 minutes under these conditions, the reactor was depressurized and shut down. The solid fraction was collected, dried at ambient temperature for 48 hours, and used in the experiments.
Microbial consortium
Two microbial consortium designated MC1 and MC2 were collected from cultivation of anaerobic hydrogen-producing bioreactor with cellulose and from sludge of a facultative pond of a paper and pulp mill Wastewater Treatment Plant (WWTP), respectively.
The bacterial communities from MC1were identified and characterized by 16S rRNA gene sequence analysis. It was mainly composed by Clostridium, Bacillus, Bacteroides and Paenibacillus genus. The sequencing data of MC1 was deposited in NCBI Sequence Read Archive under the accession number of PRJNA383576. The MC1 was cultured in Reinforced Clostridia Medium, and preserved as frozen stocks at -80°C in 50% glycerol. Before each batch test, aliquots (0.2L) of the frozen stocks were cultured in Reinforced Clostridia Medium (1.8L) for 48 hours and then used as inoculums (107CFU/mL).
The MC2 was mainly composed of Clostridium, Raoultella, Klebsiella and Desulfovibrio genus, as reported in previous study [12]. The sequencing data of the microbial consortium were deposited in NCBI Sequence Read Archive under the accession number KP715408, KP715409, KP715412 and KP715410. The MC2 was enriched in 5L Duran®flasks, in which 40% was composed of reaction volume and 60% of headspace (N2 100%). Reaction volume contained 1.8L of the enrichment medium (10g/L of yeast extract, 5g/L of tryptone and 10g/L of glucose) and 0.2L of the sludge. The initial pH was adjusted to 6.8 with HCl (1.0M). The system was incubated at 37°C for 48 hours.
Both microbial consortium were previously subjected to a total anaerobic bacteria count onto Reinforced Clostridia Medium plates (Oxoid, UK) and incubated at 37°C for 48 hours in anaerobic jars for enumeration, in order to maintain the same concentration of bacteria in each reactor (107CFU/mL).
Biohydrogen and biogas production in batch reactors
Hydrothermally pretreated sugarcane bagasse was used as substrate for the biohydrogen and biogas production through dark fermentation. This step was carried out in triplicate using1.0L batch reactors, with a 0.5L working volume constituted by the culture medium (PCS), inoculum (MC1 or MC2) and the substrate (5.0g/L). Nitrogen (N2, 100%) gas was flushed into the reactors to create anaerobic conditions. The reactors were closed with rubber stoppers and incubated at 37°C. A control assay without sugarcane bagasse was also conducted.
Culture medium used in batchreactors
PCS (peptone cellulose solution) was used as a culture medium as previously reported (Haruta et al. 2002). The constitution of the culture medium was: yeast extract (1.0g/L), peptone (5.0g/L), CaCO3 (5.0g/L) and NaCl (5.0g/L).
Analytical procedures
The biogas composition in the headspace was determined by a gas chromatography (Shimadzu GC-2010) equipped with a thermal conductivity detector using argon as the carrier gas. The temperatures of the injector, detector and column were 30°C, 200°C and 300°C, respectively. An aliquot (0.5mL) of gas samples were collected from each pressurized reactor with a pressure-lock gastight syringe. The pH and Volatile Solids Concentration (VSS) were determined in accordance with APHA (2005) [14]. Soluble carbohydrates were determined using the colorimetric phenol-sulfuric acid method [15]. The determination of Volatile Organic Acids (VFA) and alcohols was performed using a High Performance Liquid Chromatography (HPLC Shimadzu) in accordance with Penteado et al. (2013) [16].
Kinetic parameters
The experimental data was fit to the mean values of the triplicate sets of reactors using the Statistica 8.0 software. The average of the hydrogen evolution data was adjusted to the modified Gompertz model [17], which has been described as a suitable model for the adjustment of accumulated biogas production data in batch experiments [4].
In the modified Gompertz equation (Eq. 1), H is the cumulative hydrogen production, t is the time of operation (days), P is the maximum hydrogen production potential (mmol/L or mL/L), Rm is the maximum hydrogen production rate (mmol/L.day or mL/L.day), ? is the lag-phase period (day) and e is 2.71.
The hydrogen yield (mL H2/g SCB) was calculated as hydrogen production (mL/L) divided by SCB concentration added (g SCB/L).
Results and Discussion
Hydrogen production
The hydrogen production (Figure 1) from MC1 was higher (5.33mmol/L) than hydrogen production from MC2 (2.45mmol/L).
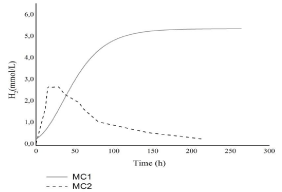
Figure 1: Hydrogen production profiles.
In the MC1, cellulose-degrading and hydrogen-producing bacteria, namely, Clostridium, Bacillus, Bacteroides and Paenibacillus, converted sugarcane bagasse into hydrogen.
Clostridium has been reported as dominant in fermentation process for hydrogen production [18]. Furthermore, many Clostridium species such as C. cellulovorans, C. drakei, C. hungatei, C. jejuense, C. aldrichii, C. carboxidivorans, C. celerecresces, C. cellulofermentans, C. cellulolyticum and C. phytofermentans are cellulolytic bacteria [19] and are able to degrade the SCB. Ho et al. also identified bacteria of the genus Clostridium from cloning and sequencing of DGGE bands in hydrogen production test from cellulosic substrates using sewage sludge as inoculum [20].
Bacillus sp. are also reported as hydrogen-producing [21,22]. Kotay and Das isolated Bacillus coagulans from aerobically digested activated sewage sludge and obtained maximal hydrogen yield (2.28mol H2/mol glucose) [23].
The Bacteroides genus has cellulose-degrading as well as hydrogen-producing species, such as B. cellulosolvens and B. xylanolyticus, respectively.
Paenibacillus sp. isolated from agricultural soils, wastewater sludge and cow dung was described as hydrogen producer [24].
In this study, the bacterial community probably was responsible for high hydrogen production from SCB. The MC1 has significant advantages over MC2, concerning the hydrogen production since there are no hydrogenotrophic microorganisms.
In the MC2, there are Clostridium, Raoutella, Klebsiella and Desulfovibrio genus. Cellulolytic bacteria, such as Clostridium sp., and non-cellulolytic bacteria, such as Klebsiella sp., can establish mutual associations in natural environments where cellulose is degraded [25]. Cavedon & Canale-Parola analyzed the interaction between these microorganisms and reported that Clostridium sp. degraded cellulose releasing soluble sugars which were used as substrate for Klebsiella sp. Meanwhile, Klebsiella sp. produced growth factors (biotin and p-amino benzoic acid) used by Clostridium sp. In our study with MC2, Clostridium and Klebsiella were probably acting syntrophically for the cellulose degradation [26].
Bacteria of the genus Desulfovibrio presents versatility in the use of carbon sources [27] and some species are capable of chemolithotrophic growth, using hydrogen as an electron donor and assimilating acetate, carbon dioxide or yeast extract as a carbon source [28]. In the present study, the substrates required for Desulfovibrio sp. may have favored the growth and maintenance of these bacteria in the reactors with MC2, and can justify the low hydrogen production.
The results of the production, rate, and yield of the hydrogen reflect the distinct abilities to degrade SCB of different microbial communities (Table 1).
P
Rate
Yield
(mmol/L)
(mL/L)
(mL/L.day)
(mL/g SCB)
MC1
5.33
119
3.8
59.5
MC2
2.45
54.9
2672.3
27.4
Table 1: Production (P), rate and yield of hydrogen from SCB.
The maximum hydrogen production (P) rate obtained with MC1was lower (3.8mL/L.day) than MC2 (2672.3mL/L.day) (Table 1). Significant difference in the production rate might be due to the fact that total hydrogen production in MC2 was reached to maximum in 1.6 days afterwards it was taken as a substrate to produce metabolites. Dark fermentation by natural microbial consortium is a process evolved to maximize the cell growth and allows the use of many substrates [7]. However, the by-products of fermentation (hydrogen, volatile fatty acids, and alcohols) are ultimately converted into methane [29].
In the literature, the number of investigations with the solid fraction of SCB hydrothermally pretreated is scarce, possibly due to the complexity of this substrate, however the hydrolysate is most commonly used [6,30].
Pattra et al. used hydrolyzed SCB without rind and small particle size (<0.5 mm) for hydrogen production by Clostridium butyricum [6]. The substrate was hydrolyzed using H2SO4 (50%) for 60 minutes at 121°C, 1.5kg/cm2 in autoclave. At these conditions, 11g glucose/L; 11.29g xylose/L; 2.22g arabinose/L; 2.48g acetic acid/L and 0.12g/L furfural were obtained in the hydrolysate of sugarcane bagasse. Hydrogen yield of 1.73mol H2/mol total sugar was obtained.
Cheng and Chang also reported high yields of hydrogen using hydrolyzate of SCB in a separate hydrolysis and fermentation process. Alkaline-pretreated bagasse was hydrolyzed by cellulolytic enzymes extracted from Pseudomonas sp. [30]. Thereafter, the bagasse hydrolysate was fermented in batch reactors at 37°C and pH 6.0 by Clostridium pasteurianum. The authors obtained a maximum H2 production of 1420.0mL/L.
The hydrogen yield obtained with MC1 was lower than the works mentioned-above, probably due to the solid fraction of pretreated SCB used as a substrate in the present study in comparison to the hydrolysate (liquid fraction of the pretreated SCB) used in the reported studies. The solid fraction has larger particles which may have affected SCB hydrolysis. The complex structure of the lignocellulosic materials creates physical and chemical barriers, making hydrolysis difficult and can justify the low hydrogen yield obtained in this condition [31].
In the present study, both the fermentation and hydrolysis proceedings were performed using microbial consortiums, highlighting the bacterial capacity to degrade SCB from fermentative metabolism.
Ratti et al. using endogenous microorganisms from unpretreated SCB as inoculum and pretreated SCB (steam explosion and alkaline delignification) as substrate (2.0g/L) obtained 7.04 mmol H2/L in comparison to 5.33 and 2.45mmol H2/L obtained in MC1 and MC2, respectively [4].
The results of the hydrogen production with MC2 (54.9ml/L) can be explained by the variety of microorganisms present in the consortium, which can produce hydrogen at a faster rate (2672.3mL/L. day). However, there is also the consumption of this hydrogen by other microorganisms, such as Desulfovibrio sp. or methanogenic archaea. Microbial consortium from environmental sources contains hydrogen consuming microorganisms, such as methanogens that consume hydrogen as electron donor and decrease its yields [29].
Methane production
Methane was produced only using MC2 as inoculum, reaching 377.66mL/g TVS after 480 hours of incubation time. Similar methane yield was obtained by Liu et al. from a SCB still age (306.97mL/g VS) after 144 hours in batch reactor at mesophilic condition (35°C) [32].
The bioconversion of lignocellulosic biomass to methane is performed by a specific microbial consortium [33], which could be composed of a large variety of microorganisms such as the producers and consumers of hydrogen which are involved in many different biochemical pathways, resulting in a complete degradation of the feedstock and improving the value-added product. Methanogenic anaerobic digestion has been recurrently performed and is advantageous over aerobic systems due to its high organic removal rates, low energy requirement, high energy production (as methane) and low sludge generation [34]. Bioconversion of lignocellulosic compounds to methane is an attractive process that has been applied around the world. At the end of the operation time (480h), the biogas content was formed by 50% of CH4 and 50% of CO2 (Figure 2).
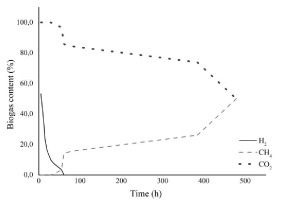
Figure 2: Biogas and hydrogen content along the time on the MC2.
Production and consumption of soluble sugars
In the reactors inoculated with MC1, the sugar concentration increased to 334.8mg/L in 29 hours with subsequent consumption. On the other hand, in MC2 assays, the soluble sugar concentration increased to 127,0 in the first 15 hours that readily consumed in 29 hours, achieving a concentration of 50.1mg/L. After this time, the soluble sugar tends to increase up to 267.3mg/L at 264 hours (Figure 3). Ratti et al. obtained similar profile with the use of microbial consortium and sugarcane bagasse as substrate from hydrogen production. The increased concentration of soluble sugars in the reaction medium can indicate the cellulolytic capability of the both culture and the use of soluble sugars to produce energy.
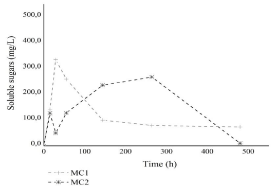
Figure 3: Concentration of soluble sugar (mg/L) in the hydrogen production
reactors.
Production of Volatile Fatty Acids
To determine the predominant metabolic pathways with the use of microbial consortium or natural microbial consortium, Volatile Fatty Acids (VFA) were measured in the samples collected at four different phases: reactor initiation, lag phase, exponential phase of hydrogen production, and at the end of each experiment (Figure 4).
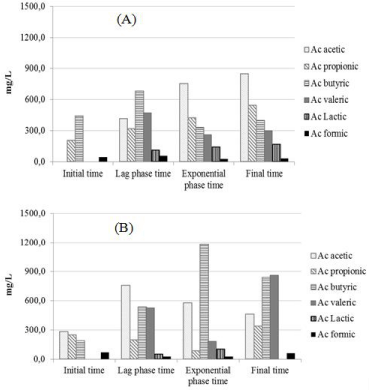
Figure 4: Concentrations of the main fermentation products observed in the
initial time, Lag phase exponential (16 hours), exponential phase time (150
hours for the MC1 and 30 hours for the MC2) and final time (480 hours) for the
final batch tests performed using SCB as substrate. MC1 (A) and MC2 (B).
VFA production was observed for both inoculum sources. Metabolites produced by MC1 reactors resulted into a higher production of acetic acid (845.7mg/L), accounting for 40% of total acids produced. Acetic acid was also the main product of cellulose, avicel and cellobiose fermentation by Clostridium sp. during hydrogen production (Ren et al.). For the SCB and others substrates, the production of acetic and butyric are preferable, according to Eqs. (2) and (3), once 4 and 2 moles of hydrogen, respectively, are formed during the fermentation [36].
C6H12O6 + 2H2O →2CH3COOH + 2CO2 + 4H2 (2)
C6H12O6→2CH CH3 CH2 COOH + 2CO + 2H2 (3)
The acetic and butyric acids totaled more than 50% of total acids produced in MC1 as well as MC2.
For MC2, the VFAs was an intermediate compound which was produced and also removed. During the time of hydrogen production with microbial consortium (initial time to exponential time), butyric (187.6 to 1184.6 mg/L) and acetic (282.3 to 1184.0 mg/L) acids were produced, whereas during the hydrogen production decline (exponential time to final time) the propionic and valeric acids were mainly produced (81.5 to 334.3 and 184.4 to 862.9 mg/L, respectively). The propionic acid has been reported in microbial consortium due to some symbiotic nature or syntrophic interactions [37].
Therefore, VFA results indicate that different metabolic pathways predominate in the presence of different cultures during SCB fermentation.
Conclusion
The hydrothermal pretreated sugarcane bagasse can be used as a substrate for hydrogen and methane production through dark fermentation. It was possible to observe the increased concentration of soluble sugars in the reaction medium which may indicate the cellulolytic capability of both cultures. Microbial consortium of Clostridium, Bacillus, Bacteroides and Paenibacillus genus can be used as a better fermentative hydrogen producer while the natural microbial consortium of paper and pulp mill WWTP is promising for methanogenic microorganisms, which can improve the overall biogas production. During the SCB conversion, the principal VFA formed by microbial consortium was acetic acid, whereas, propionic, butyric and valeric acids were also produced by the natural microbial consortium.
References
- Kapdan IK, Kargi F. Bio-hydrogen production from waste materials. Enzyme Microb Technol. 2006; 38: 569-582.
- Show KY, Lee DJ, Tay JH, Lin CY, Chang JS. Biohydrogen production: Current perspectives and the way forward. Int J Hydrogen Energy. 2012; 37: 15616-15631.
- Rabelo SC, Carrere H, Maciel Filho R, Costa AC. Production of bioethanol, methane and heat from sugarcane bagasse in a biorefinery concept. Bioresour Technol. 2011; 102: 7887-7895.
- Ratti RP, Delforno TP, Sakamoto IK, Varesche MBA. Thermophilic hydrogen production from sugarcane bagasse pretreated by steam explosion and alkaline delignification. Int J Hydrogen Energy. 2015; 40: 6296-6306.
- Das D. Advances in biohydrogen production processes: An approach towards commercialization. Int J Hydrogen Energy. 2009; 34: 7349-7357.
- Pattra S, Sangyoka S, Boonmee M, Reungsang A. Bio-hydrogen production from the fermentation of sugarcane bagasse hydrolysate by Clostridium butyricum. Int J Hydrogen Energy. 2008; 33: 5256-5265.
- Pan C, Fan Y, Hou H. Fermentative production of hydrogen from wheat Bran by mixed anaerobic cultures. Ind Eng Chem Res. 2008; 47: 5812-5818.
- Lei H, Cybulska I, Julson J. Hydrothermal Pretreatment of Lignocellulosic Biomass and Kinetics. 2013; 2013: 250-259.
- Bhat MK. Cellulases and related enzymes in biotechnology. Biotechnol Adv. 2000; 18: 355-383.
- Lay JJ. Biohydrogen generation by mesophilic anaerobic fermentation of microcrystalline cellulose. Biotechnol Bioeng. 2001; 74: 280-287.
- Temudo MF, Kleerebezem R, van Loosdrecht M. Influence of the pH on (Open) Mixed Culture Fermentation of Glucose: A Chemostat Study. Biotechnol Bioeng. 2007; 98: 69-79.
- Rabelo CABS, Botta LS, Varesche MBA. Avaliação de consórcio microbiano visando à produção de H2 a partir de celulose (Evaluation of microbial consortium aiming at the H2 production from cellulose). Proc. XI DAAL - Lat. Am. Work. Symp. Anaerob. Dig, Havana, Cuba. 2014.
- Haruta S, Cui Z, Huang Z, Li M, Ishii M, Igarashi Y. Construction of a stable microbial community with high cellulose-degradation ability. Appl Microbiol Biotechnol. 2002; 59: 529-534.
- APHA. Standard methods for the examination of water and wastewater. 21st ed. Washington, DC, USA: American Publisher Health Association. 2005.
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956; 28: 350-356.
- Penteado ED, Lazaro CZ, Sakamoto IK, Zaiat M. Influence of seed sludge and pretreatment method on hydrogen production in packed-bed anaerobic reactors. Int J Hydrogen Energy. 2013; 38: 6137-6145.
- Zwietering MH, Jongenburger L, Rombouts FM, Van’t RK. Modeling of the bacterial growth curve. Appl Environ Microbiol. 1990; 56: 1875-1881.
- Botta LS, Ratti RP, Sakamoto IK, Ramos LR, Silva EL, Varesche MBA. Bioconversion of waste office paper to hydrogen using pretreated rumen fluid inoculum. Bioprocess Biosyst Eng. 2016.
- Rainey FA. Class Clostridia. In: Brenner D, Krieg N, Staley J, editors. Bergey’s Man. Sist. Bacteriol. New York. Springer. 2009.
- Ho KL, Lee DJ, Su A, Chang JS. Biohydrogen from lignocellulosic feedstock via one-step process. Int J Hydrogen Energy. 2012; 37: 15569-15574.
- Rafrafi Y, Trably E, Hamelin J, Latrille E, Meynial-Salles I, Benomar S, et al. Sub-dominant bacteria as keystone species in microbial communities producing bio-hydrogen. Int J Hydrogen Energy. 2013; 38: 4975-4985.
- Lazaro CZ, Perna V, Etchebehere C, Varesche MBA. Sugarcane vinasse as substrate for fermentative hydrogen production: The effects of temperature and substrate concentration. Int J Hydrogen Energy. 2014; 39: 6407-6418.
- Kotay SM, Das D. Microbial hydrogen production with Bacillus coagulans IITBT S1 isolated from anaerobic sewage sludge. Bioresour Technol. 2007; 98: 1183-1190.
- Kanso S, Dasri K, Tingthong S, Watanapokasin RY. Diversity of cultivable hydrogen-producing bacteria isolated from agricultural soils, waste water sludge and cow dung. Int J Hydrogen Energy. 2011; 36: 8735-8742.
- Leschine SB. Cellulose degradation in anaerobic environments. Annu Rev Microbiol. 1995; 49: 399-426.
- Cavedon K, Canale-Parola E. Physiological interactions between a mesophilic cellulolytic Clostridium and a non-cellulolitic bacterium. FEMS Microbiol Ecol. 1992; 86: 237-245.
- Colleran E, Finnegan S, Lens P. Anaerobic treatment of sulfate-containing waste streams. Antonie Van Leeuwenhoek Int J Gen Mol Microbiol. 1995; 67: 29-46
- Kuever J, Rainey FA, Widdel F. Family Desulfuvibrionaceae. In: Brenner DJ, Krieg NR, Staley JT, editors. Bergey’s Man. Sist. Bacteriol. Vol. Two. 2nd ed., New York. Springer. 2005.
- Saady NMC. Homoacetogenesis during hydrogen production by mixed cultures dark fermentation: Unresolved challenge. Int J Hydrogen Energy. 2013; 38: 13172-13191.
- Cheng CL, Chang JS. Hydrolysis of lignocellulosic feedstock by novel cellulases originating from Pseudomonas sp. CL3 for fermentative hydrogen production. Bioresour Technol. 2011; 102: 8628-8634.
- Galbe M, Zacchi G. Pretreatment: The key to efficient utilization of lignocellulosic materials. Biomass and Bioenergy. 2012; 46: 70-78.
- Liu Y, Xu J, Zhang Y, Yuan Z, He M, Liang C, et al. Sequential bioethanol and biogas production from sugarcane bagasse based on high solids fed-batch SSF. Energy. 2015; 90: 1199-1205.
- Hendriks a. TWM, Zeeman G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol. 2009; 100: 10-18.
- Angenent LT, Karim K, Al-Dahhan MH, Wrenn BA, Domíguez-Espinosa R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004; 22: 477-485.
- Ren Z, Ward TE, Logan BE, Regan JM. Characterization of the cellulolytic and hydrogen-producing activities of six mesophilic Clostridium species. J Appl Microbiol. 2007; 103: 2258-2266.
- Antonopoulou G, Gavala HN, Skiadas I V, Angelopoulos K, Lyberatos G. Biofuels generation from sweet sorghum: Fermentative hydrogen production and anaerobic digestion of the remaining biomass. Bioresour Technol. 2008; 99: 110-119.
- Chen CC, Lin CY, Chang JS. Kinetics of hydrogen production with continuous anaerobic cultures utilizing sucrose as the limiting substrate. Appl Microbiol Biotechnol. 2001; 57: 56-64.
