1Department of Neurology, Qingdao University, China
2Department of Neurology, Shandong University, China
*Corresponding author: Hai-feng Li, Department of Neurology, Qilu Hospital (Qingdao) of Shandong University, No. 758, Hefei Road, Qingdao, China
Received: March 18, 2014; Accepted: November 22, 2014; Published: December 01, 2014
Citation: Xian-jun Zhang, Li H, Zhang Y, Zong-chao Liu and Hai-feng Li. In-Stent Thrombosis in the Middle Cerebral Artery (MCA): A Case Report. Austin J Clin Neurol 2014;1(4): 1016. ISSN : 2381-9154
In-Stent Thrombosis (IST) is a complication of angioplasty and stenting, especially in the vessels with smaller diameter. We present a case of sub acute IST after stenting treatment of isolated higher stenos is of the right Middle Cerebral Artery (MCA). Analysis of clinical features and emergent brain image helped to indicate the pathophysiological mechanism underlining the symptoms of this patient. Emergent Transcranial Doppler (TCD) helped to evaluate the compensatory collateral circulation and provided good supporting evidence in deducing the mechanism of IST. Slightly larger size of the implanted stent that caused dissection or vascular endothelium injury was presumed in this patient. Persistent evidence of inflammatory factors might also contribute to IST in this patient.
Keywords: Middle cerebral artery; Intracranial Stenosis; Angioplasty; In-stent thrombosis
In-Stent Thrombosis (IST) is a complication of angioplasty and stenting, especially in the vessels with smaller diameter. Postoperative vascular endothelium injury, collagen tissue exposure and the implanted stent may induce thrombosis [1]. Infection and inflammation may also induce impairment of vascular endothelium, leading to thrombosis. When the thrombosis cannot be recognized and treated in time, rest enosis of the artery may result. Here we report a case with IST in Middle Cerebral Artery (MCA) and discuss the process of recognizing IST and the possible mechanisms of IST in this case.
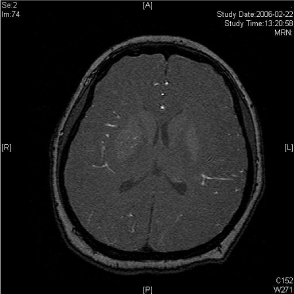
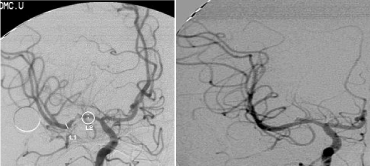
A twenty-nine-year-old female was admitted in Feb, 2006 because of episodic left limb numbness and weakness for half a year. The duration of the episode lasted for a few minutes, seldom over ten minutes. There was an attack every several days. She denied special medical history. She did not live in the pandemic area of Leptospirosis. Her blood pressure was 110/65mmHg and body weight was 51 kilograms. Physical examination found no abnormality. Magnetic Resonance Angiogram (MRA) (Feb 22) showed severe stenosis in the M1 segment of right MCA. In the source images of MRA, an oval area of abnormal signal in the right basal ganglion was found (Figure 1A). Blood routines, coagulation tests, blood biochemistry tests, and C-reactive protein level were normal. The Anti-Nuclear Antibody (ANA), anti-Extractable Nuclear Antigen (ENA) antibodies, and Anti-Neutrophil Cytoplasmic Antibodies (ANCA) were negative. Serologic test for Syphilis was negative. Blood Sedimentation Rate (ESR) was 20 mm/h, and the level of Anti- Streptolysin O microtitration test (ASO) was 395 IU/ml (0-200 IU/ ml). Digital Subtraction Angiography (DSA) detected severe stenosis (95%) in M1 segment of the right MCA, and the distal blood flow was compromised (Figure 1B). The patient was given aspirin 100mg/d, clopidogrel 75mg/d and atorvastatin 20mg/d since Feb 26. Due to the finding of high ASO level, she was given penicillin (4 million IU, bid) for a week. Because of disagreement between the consulting physician and the interventional radiologist, her angioplasty and stenting was delayed till Mar 21 on her request. During the waiting time, her attacks did not reduce. Before the operation, besides the combination of aspirin and clopidogrel, subcutaneous Low Molecular Weight Heparin (LMWH) (5000U, q12h) was given for 5 days. After a complete diagnostic evaluation of the blood vessels with DSA, stenting was performed with a stainless steel stent 2.75 mm in diameter by 1.5 cm in length (PC, Abott Company) at the pressure of 7 atm. No compromise of perforating branches was found and residual stenosis was less than ten percent on angiography (Figure 1B).
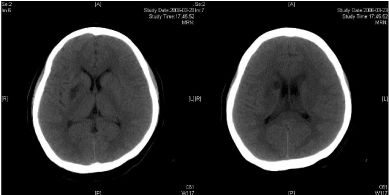
The same dose of aspirin, clopidogrel and subcutaneous LMWH was used after the operation. Two days after the stenting (Mar 23), three successive bouts of left limb weakness along with slight dizziness occurred, lasting about 10 minutes with intervals of 30 minutes. There were no abnormal signs between the first and second attacks. Babinski sign was noted during the second attack and persisted in the left between the second and third attacks with normal muscle strength, but she felt weakness. Blood pressure was 140/70 mmHg. After the third attack, her tongue deviated slightly to the left side. There was slight decrease in muscle strength and Babinski sign was positive in the left. Emergent CT displayed two low density lesions with clear edge and homogeneous density in the right basal ganglia (Figure 1C). Aspirin 300mg was given emergently. Compared with the Transcranial Doppler (TCD) test before the operation (March 3), emergent TCD showed the blood flow velocity increased significantly in both Anterior Cerebral Artery (ACA), especially in right ACA (Table 1). She got partial recovery after being given volume expansion treatment with low molecular dextran. Then she was given anticoagulant treatment with intravenous Un Fractionated Heparin (UFH) (500 U/h). LMWH was stopped. On the next day, her muscle strength recovered to normal, but pathological sign persisted. Anticoagulation therapy was continued along with dual antiplatelet therapy. On Mar 25, limb weakness appeared again, muscle strength was grade in the left, both Babinski sign and Chaddock sign were positive. Volume expansion with low molecular dextran was ineffective, and the dose of UFH was increased to750 U/h. Two days later, muscle strength and plantar reflex recovered to normal. On Mar 27, CT scan revealed that the lesion did not change compared with the previous scan, and there was no new lesion. Dose of UFH was reduced to 500H/h. During treatment, blood coagulation test was performed for several times, PT was stabled at 14.1~15.5 sec, APTT at 32.7~43.1 sec, INR at 66.6%~78.8%, activity of antithrombin (AT) at 70.4%~97.2%, and fibrinogen at 3.93~4.68 g/L. UFH was replaced by LMWH on Mar 31. CTA (Apr 3) displayed the stent was in M1 segment of right MCA, its proximal lumen was attenuated compared with the left MCA, with slightly compromised distal blood flow (Figure 1D).
She was discharged from hospital on Apr 4, and continued oral aspirin (100mg/day) and clopidogrel (75mg/day), no symptoms occurred again. TCD test on Apr 17 showed the blood flow velocity increased significantly in the right MCA, compared with that on Mar 23, the blood flow velocity in the right ICA also increased significantly but the blood flow velocity in both ACA returned to the levels before operation. TCD on May 22 showed no significant change compared with that on Apr 17. She was followed till now with few TIA attacks on aspirin only for at least five years. Four repeated ESR and ASO tests revealed persisting abnormal values, which were similar to the previous levels. TCD follow-up was similar to that on May 22, 2006.
The right MCA stenosis, which led to the Transient Ischemic Attack (TIA) symptoms, was definitely diagnosed. Angioplasty and stenting was conducted after full preparation of dual anti-platelet therapy. Immediately before the operation, LMWH was used in combination and was continued after operation. Her condition was stable in the first two days after operation. Then crescendo TIA recurred and led to motor impairment. Two lesions were shown in the emergent CT images. One was near the anterior horn of the right lateral ventricle, by which it was difficult to explain the symptoms and physical signs. The other was in basal ganglia, and was related to the symptoms and signs. The signal in this region had been slightly higher than the counterpart in the left in the source image of MRA before operation. Hence the lesion in basal ganglia was thought to result from hemodynamic impairment in distal parts of stenotic MCA. Both lesions had clear boundaries, which indicated that the infarction in the area supplied by deep branch of MCA occurred before the TIA attacks. The crescendo TIA worsened gradually, but got partial recovery after volume expansion treatment initially. TCD test showed the blood flow velocity increased in both ACA, especially in the right, while that in the right MCA did not significantly increase. This indicated that some collateral circulation had formed, to counteract hypo perfusion caused by progressive stenosis in the right MCA. Based on these, we deduced that there was IST which did not completely block the right MCA. The symptoms and physical signs appeared again when there was failure of collateral compensation. Because there were new lesions on CT scan and the patient had been treated with dual anti-platelet treatment and anticoagulation, thrombolytic therapy could not be given. So we gave Unfractionated Heparin (UFH). The symptoms and physical signs disappeared on the next day. But two days later during the same treatment, the symptoms and signs recurred, volume expansion was ineffective, this indicated that initial recovery of symptoms was due to compensatory collateral circulation, and anticoagulation therapy with UFH may also play a role. But this could not prevent thrombosis from progressing, so we increased the dose of anticoagulation drugs, the symptoms and signs disappeared eventually. CTA (Apr 3) and repeated TCD (Apr 17) confirmed our reasoning with attenuated proximal lumen of right MCA and increased blood flow velocities of the right MCA and ICA.
The intracranial angioplasty and stenting was still in its initial exploratory stage but the incidence of restenosis is higher than that of extracranial arteries. This is similar to coronary artery stenting, which is probably because that the arterial lumen diameter is smaller [1]. However, there were only individual case reports on IST in intracranial angioplasty and stenting till now. Based on the data from the study in coronary artery stenting, delayed IST were mainly related to early stopping anti-platelet drugs [2] or the poor response to anti-platelet drugs [3]. While the subacute IST is mainly related to the following three factors: 1. Histological features of stent. The incidence of IST in drug-eluting stents is lower than that in bare metal stents [4].
For thrombosis and pathological features of the lesions, especially the location and length of the lesions, anatomy of deep branch artery near the plaque.
The most well known operational factor is inadequate balloon dilation (to avoid excessive expansion of balloon which may lead to blood vessel rupture), which lead to larger residual lumen Stenosis [5]. The dissection and vascular endothelium injury caused by over-expansion or larger stent which is bigger than the lesion in diameter or length may also induce IST [6]. This patient was young, without common risk factors of cerebrovascular diseases. On screening, there was no history or evidence of connective tissue diseases and spirochete infection. The stenosis occurred in isolation in MCA. The patient was routinely given anti-platelet treatment, and was combined with anticoagulant therapy in the perioperative period. The stenosis was located in M1 segment, which was not too long, and the residual lumen stenosis was less than 10 percent. But IST was still occurred. Slightly larger size of the implanted stent that caused dissection or vascular endothelium injury was presumed. Moreover, the level of ASO and ESR was higher than normal, which persisted in the long-term. This indicated inflammatory factors might also be related to the stenosis of MCA and may induce IST. Streptococcal infection may cause dysfunction of vascular endothelial cells, which is also prone to induce thrombosis [7].
In summary, when IST is suspected, analysis of clinical features and emergent CT (or MRI) may help to indicate the pathophysiological mechanism underlining the symptoms, especially when the location and duration of the lesions and their association with symptoms are under consideration. TCD can help to evaluate the compensatory collateral circulation and provide good supporting evidence in deducing the mechanism of IST. TCD follow-ups may assess permanent restenosis and the patency of arteries in these patients. Attention should be paid to the potential factors which may induce IST.
TCD follow-ups (unit: cm/s).
Date |
L MCA |
L ICA |
L ACA |
L PCA |
L VA |
R MCA |
R ICA |
R ACA |
R PCA |
R VA |
BA |
3.3 |
104~112 |
113 |
106 |
60 |
51~58 |
266~280 |
96 |
118 |
51 |
54~59 |
83~86 |
|
144~150 |
146 |
160 |
82 |
66~72 |
359~369 |
131 |
169 |
66 |
70~76 |
107~110 |
3.23 |
109~114 |
112 |
121 |
|
|
87~91 |
93 |
161~166 |
|
|
|
|
141~145 |
152 |
168 |
|
|
110~115 |
118 |
209~212 |
|
|
|
4.17 |
93~109 |
104 |
102 |
54 |
67~69 |
160~189 |
173 |
99 |
45 |
71~76 |
78~84 |
|
119~141 |
136 |
137 |
74 |
87~88 |
202~232 |
231 |
145 |
57 |
93~97 |
98~117 |
5.22 |
88~101 |
102 |
100 |
58 |
67~69 |
157~172 |
146 |
101 |
53 |
54~58 |
77~81 |
|
113~131 |
132 |
133 |
77 |
91~93 |
194~216 |
196 |
158 |
71 |
76~78 |
101~107 |
MCA: Middle Cerebral Artery; ICA: Internal Carotid Artery; ACA: Anterior Cerebral Artery; PCA: Posterior Cerebral Artery; BA: Basal Artery. The first line of each TCD follow-up displays the average blood flow velocities, while the second line displays the systolic blood flow velocities. The range of blood flow velocities are displayed when various depth of probing is employed.
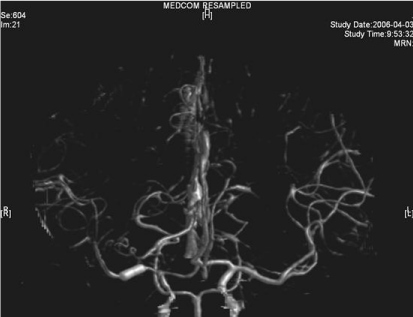
Lesion in the right basal ganglia displayed in the source image of MRA.
Right MCA before and after angioplasty and stenting.
Two infarcts displayed in CT images.
CTA image.