Case Presentation
A 67-year-old woman with a history of hypertension and Aneurysmal Subarachnoid Hemorrhage (aSAH) status-post aneurysmal clipping presented with acute onset slurred speech and left hemiparesis. Clinical examination at presentation revealed a National Institutes of Health Stroke Scale (NIHSS) of 5. Cranial Computer Tomography (CT) revealed no acute intracranial pathology. Cranial Computed Tomography Angiography (CTA) revealed no large vessel occlusion. The risks and benefits of IV alteplase administration were weighed and the patient received IV alteplase within 3.5 hours of her last known well time. The following morning the patient had improved motor strength on the left side and her dysarthria had improved with a NIHSS of 2 and repeat cranial CT revealed no interval change. The patient had an unremarkable hospital course, able to participate with physical therapy, and was discharged home.
Discussion
The safety and efficacy of thrombolytic therapy with Intravenous (IV) alteplase for acute ischemic stroke is well established. However, only a small fraction of patients receive treatment due to contraindications. The numerous contraindications for thrombolysis in acute ischemic stroke are largely based on expert opinion and not scientific evidence; in fact, the only contraindication based on a randomized clinical trial data is the time window expansion from 3 to 4.5 hours from the European Cooperative Acute Stroke Study (ECASS III) [1].
Guidelines for the use of IV alteplase from American Heart Association/American Stroke Association (AHA/ASA) have changed over the years. The AHA/ASA acute stroke management guidelines from 2013 viewed a history of previous Intracranial Hemorrhage (ICH) as exclusion for the use the IV alteplase [2]. However, a statement for healthcare professionals from the AHA/ASA from 2016 views IV alteplase in patients who have a history of ICH as potentially harmful [3]. The 2016 AHA/ASA statement does not specify if the history of aSAH would pose a risk of increased hemorrhage. However, providers at times deny alteplase for patients with acute ischemic stroke because of lack of clear distinction between intraparenchymal hemorrhage and subarachnoid hemorrhage. In addition to the guideline changes from the AHA/ASA, the Prescribing Information (PI) for alteplase has also changed [3]. In 2009 the PI for alteplase viewed a history of ICH as a contraindication for administration of IV alteplase. In 2015 the new PI for alteplase removed this contraindication and placed a warning for recent ICH [3]. Again, no comment was made in the AHA/ASA guidelines or alteplase PI if the ICH was an aSAH or if it was secured by coiling or clipping. Unfortunately, given the paucity of available safety studies or even case reports for the use of alteplase in the setting of secured aSAH makes the decision to administer IV alteplase difficulty for providers.
Several small sample size publications in recent years have observed the risk of hemorrhagic complications after off-label IV alteplase administration in patients who have contraindications to the AHA/ASA guidelines. These studies are summarized in (Table 1) [4,9]. The review of six publications reveals that in a summated total of 12 patients with history of ICH, Subarachnoid Hemorrhage (SAH), or secured SAH who received IV or Intra-Arterial (IA) alteplase, only 1 of the 12 patients had a symptomatic ICH (SICH). Given this small sample size, it is clear that further observational studies need to be publish and randomized clinical trials need to be performed to assess the risk of administering IV or IA alteplase in the setting of a patient with history of ICH, both parenchymal and secured aneurysmal. Current guidelines do not specify the etiology of ICH as aneurysmal or parenchymal and do not comment if aneurysmal causes of ICH have been secured by coiling or clipping.
Furthermore, not all secured aneurysms are in fact secured and this should be considered in the use of IV alteplase in patients who have secured aSAH. It is well known that coiled aneurysms require extended imaging monitoring [10]. According to AHA/ASA, the use of IV alteplase in the setting of unruptured and unsecured intracranial aneurysms has established guidelines. The AHA/ASA recommends that it is reasonable and probably recommended that patient who have unsecured and unruptured aneurysms that are less than 10mm in size to receive IV alteplase [3]. No recommendations are established for patients who have giant unruptured and unsecured intracranial aneurysms [3].
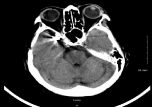
Figure 1: Cranial Computer Tomography (CT) of patient in case report
showing clipping of MCA aneurysm.
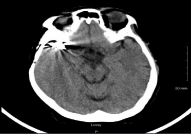
Figure 2: Cranial Computer Tomography (CT) of patient in case report
showing clipping of MCA aneurysm.
Sample Size (N)
Intracranial Lesion
Therapy Administered
SICH After IVT
Reference
3
ICH or SAH
IVT
0
[4]
3
ICH
IVT
1
[5]
1
ICH
IVT
1
[6]
3
Secured SAH
IVT
0
[7]
1
Secured SAH
IVT
0
[8]
1
Secured SAH
IAT
0
[9]
Table 1: ICH: Intracranial Hemorrhage; SAH: Subarachnoid Hemorrhage; IVT: Intravenous thrombolysis; IAT: Intra-Arterial Thrombolysis; SICH: Symptomatic Intracranial Hemorrhage; Secured SAH:
We encountered a patient with a history of aSAH that was secured a decade prior with clipping who presented for concern of acute ischemic stroke, clipping is show in (Figures 1, 2). The management strategy involved weighing the risks and benefits of IV alteplase administration in a patient with history of secured aSAH. Our patient had a history of right middle cerebral artery bifurcation aneurysmal SAH with clipping of the aneurysm, with seven clips in fact, a decade ago. After IV alteplase administration, our patient's CT head did not reveal a new or symptomatic ICH nor did it reveal evidence of an ischemic stroke. We concluded that our patient likely had an exacerbation of her prior stroke symptoms. Nonetheless, a patient with a history of secured aSAH was administered IV alteplase without a negative outcome.
According to current AHA/ASA guidelines, administering IV alteplase in a patient with history of ICH is considered potentially harmful and IV alteplase is reasonable and probably recommended in patients with unsecured and unruptured aneurysms less than 10mm in size [3]. However, we argue that a patient with a history of secured aSAH that had the aneurysm secured with either coiling or clipping should be considered strongly for IV alteplase in the setting of concern for acute ischemic stroke. The risks and benefits regarding the administration of IV alteplase should be tailored for each patient. However, patients who have a history of secured aSAH should be considered strongly for the use of IV alteplase as the current body of observational studies to date reports a low frequency of spontaneous ICH after IV alteplase administration.
Conclusion
Current guidelines consider IV alteplase administration in patients with a history of ICH potentially harmful and previously were a contraindication to IV alteplase administration. Over the last decade observation studies have found that patients with a history of ICH can be administered IV alteplase with a low frequency of spontaneous ICH, which we also observed in our patient presented herein. We recommend considering strongly IV alteplase in patients with a history of aneurysmal ICH that has been secured with coiling or clipping in the setting of concern for an acute ischemic stroke.
References
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. New England Journal of Medicine. 2008; 359: 1317-1329.
- Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Demaerschalk BM, et al. American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013; 44: 870-947.
- Demaerschalk BM, Kleindorfer DO, Adeoye OM, Demchuk AM, Fugate JE, Grotta JC, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016; 47: 581-641.
- Meretoja A, Putaala J, Tatlisumak T, Atula S, Artto V, Curtze S, et al. Off-label thrombolysis is not associated with pooroutcome in patients with stroke. Stroke. 2010; 41: 1450-1458.
- Kvistad CE, Logallo N, Thomassen L, Waje-Andreassen U, Brøgger J, Naess H. et al. Safety of off-label stroke treatment with tissue plasminogen activator. ActaNeurologicaScandinavica. 2013; 128: 48-53.
- Buchan AM, Barber PA, Newcommon N, Karbalai HG, Demchuk AM, Hoyte KM, et al. Effectiveness of t-PA in acute ischemic stroke: outcome relates to appropriateness. Neurology. 2000; 54: 679-684.
- Aleu A, Mellado P, Lichy C, Köhrmann M, Schellinger PD. Hemorrhagic complications after off-label thrombolysis for ischemic stroke. Stroke. 2007; 38: 417-422.
- Kutlu R, Alkan A, Kocak A, Sarac K. Thrombolysis and Mechanical Fragmentation to Treat Massive Pulmonary Embolism in a Patient with an Anterior Communicating Artery Aneurysm. Journal of Endovascular Therapy. 2003; 10: 332-335.
- McLaughlin N, Bojanowski MW. Intra-arterial thrombolys is following clipping of an unruptured aneurysm. Canadian Journal of Neurological Sciences. 2008; 35: 672-673.
- Williams LN, Brown RD. Management of unruptured intracranial aneurysms. Neurology Clinical Practice. 2013; 3: 99-108.
