Abstract
Introduction: Pseudocyst formation and pancreaticopleural fistula are a rare complication of acute and chronic pancreatitis.
Case Presentation: A 10-year-old girl was admitted to our paediatric emergency department with an acute onset of dyspnoea. On physical examination, she was found to be dyspnoeic and tachypnoeic and having dullness to percussion with decreased breath sounds on the left hemithorax. Chest X-ray, computed tomography and magnetic resonance imaging demonstrate the existence of a multilocular lesion in the left hemithorax region that compresses the mediastinum and pushes it to the right position, pancreas with calcium zones and a liquid zone that can correspond to a pancreas pseudocyst. The patient was successfully treated with combined operative (thoracotomy), invasive (percutaneous puncture of pseudocysts of pancreas) and conservative therapy.
Conclusion: Pancreaticopleural Fistula (PPF) is an uncommon complication of chronic pancreatitis leading to large and recurrent pleural effusion. The condition is diagnosed with very high pleural fluid amylase, Rtg and CT. We recommend a conservative treatment of chronic pancreatitis in combination with percutaneous drainage of pseudocysts of the pancreas, thus reducing the accumulation of fluid in the torax.
Keywords: Pancreaticopleural Fistula; Pseudocyst; Pancreas
Introduction
Pseudocyst formation and pancreaticopleural fistula are a rare complication of acute and chronic pancreatitis. These cysts are located inside and around the pancreas. Pancreaticopleural Fistula (PPF) has been recognized as a clinical entity since case reports were published in late 1960s [1]. PPF occurs in 0.4% of patients with pancreatitis and 4.5% of patients with pancreatic pseudo cysts. For children, pancreatic diseases are rare and the exact incidence of PPF is not known [2].
Case Presentation
A 10-year-old girl was admitted to our paediatric emergency department with an acute onset of dyspnoea. On physical examination, she was found to be dyspnoeic and tachypnoeic and having dullness to percussion with decreased breath sounds on the left hemithorax.
The trouble began 10 days before admission to our Clinic. The patient had an elevated body temperature and cough. The patient was treated in a competent hospital and then at the Pulmonary Pediatric Clinic where antibiotics Longacef and Amikacin were administered. A chest X-ray showed left side pleural effusion (Figure 1).
Chest CT is done to demonstrate the existence of a multilocular lesion in the left hemithorax region that compresses the mediastinum and pushes it to the right position. CT shows a changed appearance of pancreas with calcium zones. There is a liquid zone that can correspond to a pancreas pseudocyst. We found the liquid zone that can match pseudocyst of the pancreas which has a diameter of up to 20cm (Figure 2, 3).
Although we noticed changes in the pancreas and cystic zone in the abdominal cavity below the left diaphragm with similar CT density, we did not think it was a PPF. We performed thoracoscopy first and we saw a blurred pleural fluid and a visceral and parietal pleura covered with fibrin deposits (Figure 4). We have set an emergency thoracotomy indication for severe respiratory status and moving the mediastinum to the opposite side. We only suspected on PPF when we received an analysis of the amylase value in the pleural punctate. Due to hypertensive liquidotorax, urgent thoracotomy was performed and we evacuatied about 2 liters of serohemorrhagic fluid and left 2 thoracic tubes.
Pleural fluid cytology was negative for malignant cells, and the pleural fluid culture was negative. Levels of amylase in pleural fluid (16810IU/L), serum (1250IU/L; normal 30-110IU /L) and urine (2300IU/L; normal 32-641IU/L) were elevated. After surgery, the toracotomy patient was located in the intensive care unit. The patient was conservatively treated by a pediatric gastroenterologist with total parenteral nutrition. There is a great secretion on thoracic drainage for the first 10 days. Percutaneous drainage of pseudocyst pancreas was indicated in order to reduce secretion to thoracic drains. After draining the pseudocyst pancreas, the secretion to the thoracic tubes were reduced and completely disappeared, and the thoracic tubes were removed. MRCP was performed to show dilated both pancreatic ducts up to 7 mm with pancreatolytes (Figure 5).
A conservative treatment of chronic pancreatitis was continued by a pediatric gastroenterologist. The fatty diet was regulated with addition of the enteral formula Fresubine Energy drink 3x200ml orally. We have considered the possibility of invasive procedures such as pancreatic duct stenting or surgery in case of failure of conservative treatment. The values of amylases in the blood are reduced. The splitting of the percutaneous pseudocyst completely stopped 2 weeks after the application. The control findings of the Echo abdomen cannot verify the existence of a pseudocystic formation. Medical conservative treatment resulted in spontaneous closure of the fistula and surgical intervention was not required. She was discharged from the hospital on the 33th day of hospitalization.
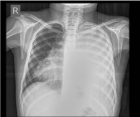
Figure 1: A chest X-ray showed left side pleural effusion.
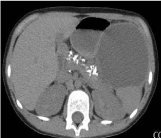
Figure 2: CT- pancreas with calcium zones and pancreas pseudocyst.
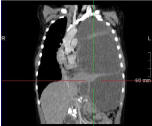
Figure 3: CT - multilocular lesion in the left hemithorax which pushes the
mediastinum in to the right position.
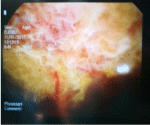
Figure 4: Thoracoscopy - blurred pleural fluid and visceral and parietal pleura
covered with fibrin deposits.
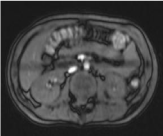
Figure 5: MRCP - dilated both pancreatic ducts up to 7mm with
pancreatolytes.
Discusion
The effusion in chronic pancreatitis and PPF is usually left sided, but may even be right sided or bilateral [3]. Secondary to the chronic inflammation of the pancreas, the main pancreatic duct is obstructed and ruptured [4]. Pancreatic duct disruption develops secondary during the course of chronic pancreatitis in children [5]. If the ductal leakage is anterior, pancreatic secretions flow freely into the abdominal cavity as pancreatic as cites occurs [6]. If it is posterior, the retroperitoneal pancreatic fluid tends to go towards the mediastinum because of the transdiaphragmatic pressure gradient between the abdominal and pleural cavities. The fluid uses the esophageal or aortic hiatus as a migration path [7].
According to the leakage of the pancreatic fluid in chest termination sites, a pancreatic fistula can be divided into four types: A pancreatic pleural fistula, a pancreatic mediastinum fistula, a pancreatic broncho fistula and a pancreatic pericardium fistula. PPF occurs in 0.4% of patients with pancreatitis and 4.5% of patients with pancreatic pseudo cysts [8]. Pancreatic diseases are rare for children and the exact incidence of PPF is not known. Chronic pancreatitis is result of biliary duct obstruction constitutes the major etiological factor in children. Only a few congenital malformations have been reported; these include congenital pancreatic outflow obstruction and pancreatic separation [9]. Trauma contributes 0.5% of cases. Nearly all of the cases reported in children were associated with pancreatic pseudo cysts [10].
The symptoms in PPF include respiratory difficulties, chest pain and bloody pleural effusion. Only a small portion of patients suffer from abdominal pain. The most common symptom is dyspnea, accounting for 65-67% of the reported cases [11].
Pancreatic fistula is usually expressed in clinical manifestations of hemorrhagic pleural effusion together with an increase in the amylase. Amylase level is not a routine examination in pleural effusion; thus, it is often missed and diagnosis is delayed. The diagnosis of PPF may be explicit if levels of amylase in the pleural effusion are greater than 5000U/L.
CT, ERCP and MRCP are most widely used in current practice, and the sensitivity of each modality in detecting PPF is 47%, 78% and 80%, respectively. CT is very valuable for the diagnosis of this disease and for concurrently identifying the pleural effusion and mediastinal pseudocysts and whether an existing pancreatic pseudocyst is present and displaying the part of fistula. Endoscopic Retrograde Cholangiopancreatography (ERCP), which can clearly show pancreatic damage and fistula, used to be the preferred method of investigation for confirming the diagnosis of PPF. Magnetic Resonance Cholangiopancreatography (MRCP) is a noninvasive procedure compared to ERCP and has superiority over CT in that it can directly show the relationship between the pancreatic duct and fistula by clearly showing the base position of a pancreatic cyst and fistula [12].
Conservative treatment of PPF is aimed at suppressing pancreatic activity to promote the resolution of the pleural effusion and closure of the fistula, including pleural effusion drainage, parenteral nutrition and somatostatin analogue. Endoscopic treatment of transpapillary stents or NPD (nasopancreatogram) placement should be the therapy of choice for patients with PPF. Surgical repair should be reserved for patients who fail endoscopic therapy. [13]. Pancreatic resection and enteropancreatic anastomosis constitute methods of surgical intervention to achieve drainage of pancreatic secretions.
Comparing the literature experience with our case, we decided to apply urgent thoracotomy and drainage due to the acute condition in thorax and the movement of the mediastinum into the contralateral side. After that, we decided to reanimate the patient with parenteral and enteral support (gastroenterological treatment of chronic pancreatitis). We decided to performe percutaneous drainage of the pericidal cavity in PPF, in order to reduce secretion to thoracic drains and reduce the need for them, thus additionally mobilizing the patient. We did not find this procedure in the literature on the treatment of PPF and we consider it to be very useful. Fortunately, conservative treatment gave results and thus we did not have the need for endoscopic or surgical treatment of chronic pancreatitis and pancreatolithiasis.
Conclusion
Pancreaticopleural Fistula (PPF) is an uncommon complication of chronic pancreatitis leading to large and recurrent pleural effusion. The condition is diagnosed with very high pleural fluid amylase, Rtg and CT. We recommend a conservative treatment of chronic pancreatitis in combination with percutaneous drainage of pseudocysts of the pancreas, thus reducing the accumulation of fluid in the torax
References
- Ozbek S, Gumus M, Yuksekkaya HA, Batur A. An unexpected cause of pleural effusion in paediatric emergency medicine. BMJ Case. 2013.
- Duncan ND, Ramphal PS, Dundas SE, Gandreti NK, Robinson-Bridgewater LA, Plummer JM, et al. Pancreaticopleural fistula: A rare thoracic complication of pancreatic duct disruption. J Pediatr Surg. 2006; 41: 580-582.
- Takeda N, Kitano Y, Honna T. Bilateral pancreaticopleural fistula in an infant: A rare complication of chronic relapsing pancreatitis. Med J. 2013; 43: 151-154.
- Sonoda S, Taniguchi M, Sato T, Yamasaki M, Enjoji M, Mae S, et al. Bilateral pleural fluid caused by a pancreaticopleural fistula requiring surgical treatment. Intern Med. 2012; 51: 2655-2661.
- Ranuh R, Ditchfield M, Clarnette T. Surgical management of a pancreaticopleural fistula in a child with chronic pancreatitis. J Pediatr Surg. 2005; 40: 1810-1812.
- Drescher R, Köster O, Lukas C. Mediastinal pancreatic pseudocyst with isolated thoracic symptoms: A case report. Journal of Medical Case Reports. 2008; 2:180.
- Komtong S, Chanatrirattanapan R, Kongkam P, Rerknimitr R, Kullavanijaya P. Mediastinal pseudocyst with pericardial effusion and dysphagia treated by endoscopic drainage. JOP. 2006; 7: 405-410.
- Bhasin DK, Rana SS, Chandail VS, Nanda M, Sinha SK, Nagi B, et al. Successful resolution of a mediastinal pseudocyst and pancreatic pleural effusion by endoscopic nasopancreatic drainage. JOP. 2005; 6: 359-364.
- Sadat U, Jah A, Huguet E. Mediastinal extension of a complicated pancreatic pseudocyst: A case report and literature review. J Med Case Reports. 2007; 1: 12.
- Groeneveld JH, Tjong A, Lieng JG, de Meijer PH. Resolution of a complex mediastinal pseudocyst in a patient with alcohol related chronic pancreatitis following abstinence from alcohol. Eur J Gastroenterol Hepatol. 2006; 18: 111-113.
- Saftoiu A, Ciurea T, Dumitrescu D, Stoica Z. Endoscopic ultrasound-guided transesophageal drainage of a mediastinal pancreatic pseudocyst. Endoscopy. 2006; 38: 538-539.
- Xiang Q, Zheng F. Pancreatic pleural fistula due to a pancreatic cyst in a child: A case report. Biomedical Research. 2017; 28: 4705-4708.
- Bhasin DK, Rana SS. Response to endoscopic therapy as a first-line therapy for pancreatic-pleural fistula. Surgery. 2010; 148: 609-610.
