Abstract
Fat Embolism Syndrome (FES) is the systemic manifestation of fat emboli in the circulation-a rare and potentially lethal complication of trauma, seen mostly after long bone fractures. Hypoxia is the most common and earliest feature of FES, followed by Central Nervous System manifestations. Other than supportive treatment, no exclusive and defined treatment approach has yet been identified. In the ICU, there has been an increased use of High Flow Nasal Cannula Oxygen (HFNC) in situations of acute respiratory failure. In the case under analysis, respiratory failure with hypoxemia occurs in a young patient with risk factors for fat pulmonary embolism, who responded well to supportive care.
Keywords: Hypoxia; Fat embolism; Tibial Factures; Non-Invasive Ventilation; Lung Injury
Abbreviations
FES: Fat Embolism Syndrome; FE: Fat Embolism; HFNC: High Flow Nasal Cannula Oxygen
Introduction
FES is an infrequent complication related to long bone fracture with a constellation of signs and symptoms depending whether there is systemic evolvement or solely lung injury with marked hypoxia and need for respiratory support. Diagnosis is mainly clinical with no formal standard treatment and delayed approach may have devastating consequences. We report a case of Fat Embolism (FE) after delayed reaming of a tibial fracture, evolving to respiratory failure and need for non-invasive ventilation. An overview of the literature and discussion of the features is provided.
Case Report
An 18-year-old male patient with a history of asthma was presented to the emergency room after a high-speed injury. Despite initial loss of consciousness, neurologic findings were normal on arrival. First clinical findings revealed right leg trauma with limb edema, hemodynamic stability, and no respiratory alterations. Head CT-scan was normal and radiography of right leg showed a tibial fracture. Limb immobilization was performed and 3 days after admission the patient underwent surgical intervention with closed reduction and reaming of the right tibia.
Shortly after surgery, he developed symptoms of chest pain and dyspnoea. The patient was hemodynamically stable with a heart rate of 109bpm, respiratory rate of 30cpm and oxygen saturation of 93% with oxygen therapy of 6 L/min through facial mask. Arterial blood gas, on 6L/min of oxygen, revealed pH 7.43, pO2 60.1 mmHg, pCO2 39 mmHg, Sat O2: 91%, HCO3 26.4 mmol/L, Lactate 2.2 mmol/L, PaO2/FiO2 of 136. There was no evidence of external bleeding or circulatory instability. Chest radiography revealed bilateral interstitial infiltrates (Figure 1), followed by CT-Angiography of the chest that ruled out pulmonary thromboembolism, but revealed extensive ground glass opacities (Figures 2A,2B,2C). Due to acute respiratory distress, the patient was admitted to the ICU, followed by non-invasive respiratory support with HFNC with significant improvement. Blood analysis showed: Hemoglobin 9.8g/dL, without leucocytosis, platelets 132000 U/L, Dimers of 1217 U/L, Calcium of 7.7mmol/L, LDH 404 U/L, CK 2855 U/L and a myoglobin of 831.5 ng/mL. Due to the unclear etiology of the CT scan findings, he was started on empiric antibiotics, stopped 24h later, due to noncompelling evidence of infection. Etiological investigation ensued: cultural exams (blood cultures, Mycoplasma and Chlamydia blood antigens, Legionella and Pneumococcus urinary antigens), serologies (HIV 1-2), and auto-immunity studies (Anti-citrulline, Rheumatoid factor, Anti-neutrophil cytoplasm, Anti-MBG, Antinuclear, antidsDNA, Anti-nucleosome antibodies and ENA), came back negative. Bronchofibroscopy with bronchoalveolar lavage cytology revealed innumerous lipid-laden macrophages-alveolar macrophages with cytoplasmic fat inclusions (Figures 3,4). Oil Red O stains neutral triglycerides and lipids, indicating lipid accumulation in the cytoplasm of alveolar macrophages. The diagnoses of FE after nailing of isolated right tibial fracture was assumed, with favourable radiological evolution and almost complete clearance of infiltrates by day 5. Patient was weaned from HFNC after 3 days and later transferred to the orthopedics ward, with an uneventful hospitalization.
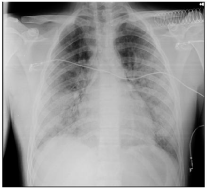
Figure 1: Chest Radiography with bilateral infiltrates.
Discussion
FES is a complication following traumatic injuries (such as long bone and pelvic fractures), cardiopulmonary resuscitation. Nontraumatic conditions (acute pancreatitis, fatty liver, corticosteroid therapy, fat emulsion infusion, and hemoglobinopathies are rare causes of FES [1-3]. Actual incidence of FES is unknown. In patients with long bone fractures, FES incidence has been reported to occur in 0.5 to 11% [2]. It is seen more frequently in closed than open fractures, increasing risk in proportion with the number of bone structures involved [4]. Despite advances in diagnostic techniques and increased understanding of the pathological processes that lead to fat embolization, the clinical correlate, known as Fat Embolism Syndrome (FES), remains poorly understood. A fat embolism is the presence of fat within vascular structures. The Fat Embolism Syndrome (FES) constitutes a potentially devastating constellation of clinical signs and symptoms, classically as the triad of respiratory insufficiency, neurologic dysfunction, and petechial rash [1-5]. There are no universal criteria for diagnosis, management, or laboratory tests. FES usually manifests as a multisystem disorder with an asymptomatic interval of about 12-72 hours after initial insult, followed by a classical triad of respiratory insufficiency, neurologic manifestations and petechial rash. Pulmonary manifestation is the earliest symptom, seen in 75% of patients [6]. Symptoms vary from dyspnea, tachypnea, and hypoxemia to ARDS. Hypoxia is the most common finding (96% of patients). Other nonspecific symptoms include fever, thrombocytopenia, jaundice, lipuria, haematuria, and retinopathy [3]. Our patient presented with dyspnoea, tachypnea, tachycardia, and hypoxemia, Diagnosis is made by clinical suspicion and suggestive findings on imaging methods. On CT scan, includes areas of consolidation, ground-glass opacities (Figures 2A, 2B,2C), small nodules or fat attenuating filling defects in pulmonary arteries. Laboratory findings are nonspecific. Some patients may develop thrombocytopenia, anemia, or even hypofibrinogenemia. Cytological examination of the urine and sputum may show fat globules, but their diagnostic role still remains controversial [6]. Lipid inclusion in the macrophages might point to FES, despite not specific, as this can be seen in other conditions [5-6]. Due to the fact that it is invasive and time-consuming, it is seldom used in the diagnosis. Mainstay of treatment for FES is supportive [7,8]. Prevention, early diagnosis, and adequate symptom management are paramount.
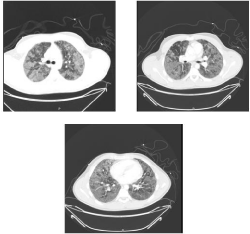
Figure 2A,2B,2C: CT-Angiography showing parenchymal ground glass opacities with no signs of vascular embolism.
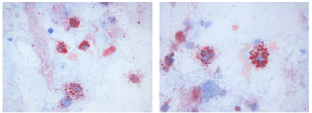
Figure 3,4: Microscopy Imaging of Bronchoalveolar Lavage Cytology (Oil Red O, 400x-750x).
Respiratory failure is characterized as permeability edema with decreased compliance similar to oleic acid lung injury [8]. Gas exchange abnormalities include shunt and increased dead space from atelectasis and alveolar flooding comparable to Acute Lung Injury (ALI) and ARDS from other causes [8-10].
The use of HFNC in the ICU represents an important alternative in non-invasive ventilatory support for these patients with advantages such as higher flow rates, which create a positive pressure effect and reduces the anatomic dead space, delivering a predictable and constant FiO2, increasing the partial arterial pressure of oxygen PaO2/FIO2 ratio which reduces the entrainment of room air and the dilution of oxygen, providing heated and humidified gas that is inhaled, improvement of mucociliary motion and sputum clearance; reducing upper airway resistance and work of breathing; and improving thoraco-abdominal synchrony. Studies have found HFNC had an excellent tolerability, increased oxygenation and decreased the dyspnea score in adult patients. Studies have also demonstrated a good clinical response with the use of this technique in Pulmonary Embolism (PE) [10].
In PE, ventilation increases intrathoracic pressure which may result in decreased right stroke volume and arterial pressure. Although it has been shown to increase end-expiratory lung volume, HFNC’s positive pressure is mainly observed during expiration [10]. In this case, HFNC successfully avoided complications of invasive ventilation and contributed to a quicker response being superior to non-invasive ventilation by not increasing intrathoracic pressure and reducing upper airway resistance. After 3 days, the patient had a full recovery and no further oxygen needs. However, most of the studies addressing these applications are of low quality, limiting the ability to make recommendations. Further studies are needed to confirm the clinical advantages of HFNC over other modalities, to evaluate longer-term effects and to determine its optimal use.
Conclusion
Most of the morbidity associated with FES is related to pulmonary dysfunction. However, pulmonary presentations of fulminant FES in a multitrauma patient can be identical to the presentation of ARDS. It may be impossible to differentiate and establish the etiology of ARDS/ ALI. Investigators agree that with the improvement in supportive therapy and early stabilization and definitive fixation of fractures, the overall outcomes of patients with FES has improved. HFNC can be an important tool in the management of oxygen supply in these patients.
References
- Uransilp N, Muengtaweepongsa S, Chanalithichai N, Tammachote N. Fat Embolism Syndrome: A Case Report and Review Literature, Hindawi Case Reports in Medicine. 2018; 1.
- Aparicio G, Soler I, Lopez-Duran L. Fat embolism syndrome after nailing an isolated open tibial fracture in a stable patient: a case report, BMC Research Notes. 2014; 7: 237.
- Grigorakos L, Nikolopoulos I, Stratouli S, Alexopoulou A, Nikolaidis E, Fotiou E, et al. Fat Embolism Syndrome-Three Case Reports and Review of the Literature, Journal of Trauma Injury. 2017; 30: 107-111.
- Sotello D, Rivas M, Mulkey Z, Nugent K. High-Flow Nasal Cannula Oxygen in Adult Patients: A Narrative Review, American Journal of the Medical Sciences, February. 2015; 349: 179-185.
- Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome, Injury. Int J Care Injured. 2006; 37: S68-S73.
- Kwiatt ME, Seamon MJ. Fat embolism syndrome, International Journal of Critical Illness and Injury Science. 2013; 3: 64-68.
- Shaikh N. Emergency management of fat embolism syndrome, Journal of Emergency Trauma Shock. 2009; 2: 29-33.
- Lee JH, Park JH, Kim YK, Kim JW, Kim YC, Kim H, et al. A case of fat embolism syndrome complicated by diffuse alveolar hemorrhage, Hong Kong Journal of Emergency Medicine. 2018; 25: 102-105.
- Mellor A, Soni N. Fat embolism Anaesthesia, 2001; 56: 145-154.
- Roca O, Messika J, Caralt B, Garcia-de-Acilu M, Sztrymf B, Ricard JD, et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: The utility of the ROX index, Journal of Critical Care. 2016; 35: 200-205.
