Abstract
Mullerian adenosarcoma of the endometrium in adolescent girls is extremely rare, with only fifteen cases under 20 years old having been reported to date. We describe here a new case of adolescent Mullerian adenosarcoma and provide an updated review of the previous literature on such rare tumors. Our 19-year-old case presented with a six-month history of prolonged menstruation. She had not yet had any sexual relationship. On gross examination, a fragile mass was seen in her vagina that bled easily. A 4.0×2.0 cm mass was visualized with Magnetic Resonance Imaging (MRI). The tumor seemed to slightly invade the myometrium of the uterine corpus. Transvaginal ultrasound sonography confirmed the presence of a 4.0 cm mass located in the cervix and vagina. The tumor biopsy was diagnosed as a Mullerian adenosarcoma of the endometrium. We performed a Total Abdominal Hysterectomy (TAH) and Bilateral Salpingectomy (BS). The post-surgical specimen was diagnosed as a pT1aNXM0 Mullerian adenosarcoma of the endometrium. The patient did not require adjuvant chemotherapy. She has been monitored every 3 months and has been without recurrence now for 28 months.
Keywords: Mullerian Adenosarcoma; Endometrium; Adolescent Girl; Fertility
Introduction
First reported by Clement and Scully in 1974 [1], Mullerian adenosarcoma was described as a distinct histologic type of the mixed mesodermal tumors. This rare new tumor was considered to be a mesodermal tumor consisting of benign glandular epithelial cells and malignant mesenchymal cells, it now represents nearly 8% of all uterine sarcomas. Mullerian adenosarcomas occur most frequently in postmenopausal women; their occurrence in adolescent girls is quite rare. In adolescent girls, there are a few reports of Mullerian adenosarcoma of the cervix but Mullerian adenosarcomas of the endometrium are extremely rare. To our knowledge, only 15 cases under 20 years old occurrences [2-16] have been reported since the time of Clement and Scully’s first definition of it; these reports are summarized in (Table 1). We report here on a new case of Mullerian adenosarcoma of the endometrium in a 19-year-old girl, and we provide an updated literature review concerning all previous such cases.
Case Presentation
A 19-year-old girl presented to her local hospital with a six-month history of prolonged menstruation. She denied having had a sexual relationship. On gross examination, an easily-bleeding fragile mass was seen in her vagina. The patient’s CA125, CA19-9, and CEA serum levels were normal. A 4.0×2.0 cm mass attached to endometrium was seen by Magnetic Resonance Imaging (MRI). The tumor seemed to slightly invade the myometrium of the uterus corpus (Figure 1). A transvaginal ultrasound and biopsy were performed under anesthesia. Sonography confirmed the presence of a 4.0 cm mass located in the cervix and vagina but it could not confirm its exact origination. It was difficult to see the stem of the mass and the cervix because of the persistent bleeding.
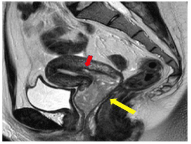
Figure 1: MRI image before surgery (T2 weighted, sagittal section). A 4 cm
mass (yellow arrow) was located in the cervix of the uterus. The mass was
suspected to invade the myometrium slightly (red arrow).
Histological examination showed normal endometrial glands pushed to the slide by proliferative stroma, causing the shapes of the glands to appear dented and curved. A prototypical hypercellular ‘periglandular-cuffing’ pattern was observed around the glands (Figure 2). Nuclear atypia was specifically observed only in the stromal cells; the glandular epithelium was devoid of nuclear atypia. In (Figure 3), the tumor cells were negative by immunohistochemistry for desmin, a tumor marker for smooth muscle cells. In (Figure 4), the tumor was positive for overexpression of p16 and Ki67 and was still negative for desmin. The tumor was negative for CD10 in the stromal cells but positive around the glandular epithelium. The tumor was diagnosed as a Mullerian adenosarcoma of the endometrium.
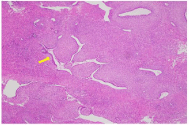
Figure 2: H&E staining of the tumor (×40). Normal endometrial glands
seemed to be pushed away by the pressure of the proliferative stroma and the
shapes of the glands were dented and curved. A hypercellular ‘periglandular
cuffing’ pattern (arrow) was observed around the glands.
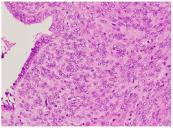
Figure 3: H&E staining of the tumor (×400). The glandular epithelium
displays no nuclear atypia; the nuclear atypia was specifically observed in
the stromal cells.
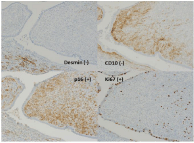
Figure 4: MRI image before surgery (T2 weighted, sagittal section). A 4 cm
mass (yellow arrow) was located in the cervix of the uterus. The mass was
suspected to invade the myometrium slightly (red arrow).
The patient and her family were informed of the diagnosis and given options and risks for treatment. On whole-body PET-CT, there was no abnormal uptake of tracer indicative of malignancy elsewhere. At this point, we suspected that the mass was an aggressive adenosarcoma of the endometrium with some invasion into the myometrium because on MRI the stem of the mass seemed to be developed from the myometrium of the uterine corpus. This led us to believe that it would be difficult to preserve her uterus, but the available literature suggested that it might be possible to preserve her ovaries. We offered a Total Abdominal Hysterectomy (TAH) because we thought it would be too difficult to completely remove only the tumor, considering its invasion into the myometrium. We discussed with her and her family that if they desired fertility-preserving surgery instead of hysterectomy, we could attempt resection, although the surgery might leave residual tumor cells, leading to poorer prognosis. After a lengthy discussion with the patient and her family, we all agreed to attempt to perform TAH and Bilateral Salpingectomy (BS), but with preservation of the ovaries. During the laparotomy, a minor amount of serous ascites was observed in the pelvic cavity. Both ovaries, fallopian tubes, and uterus were normal in appearance. There were no other lesions indicative of dissemination or endometriosis. An uncomlicated TAH and BS were performed.
Upon post-surgical histological examination of the removed uterus, we found only a raised 2mm in diameter in the endometrium of the uterus, this was because most of the mass was removed by the pre-surgical biopsy (Figure 5). We couldn’t see the tumor invasion to the myometrium macroscopically. By H&E staining, we couldn’t clearly see the tumor. By immunohistochemistry (Figure 6), we could determine that the tumor cells that invaded the myometrium were negative for desmin and CD10, and positive for the overexpression of p16, which was the same staining pattern we saw in the presurgical biopsy, from which we diagnosed the tumor as a Mullerian adenosarcoma of the endometrium (pT1aNXM0). The pathologic diagnosis of the ascites was negative. The patient did not require adjuvant chemotherapy. She has been monitored with follow-up visits every 3 months since the surgery and has been without any signs of recurrence for 28 months.
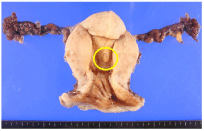
Figure 5: Resected uterus and fallopian tubes only a raised nodule (circle)
existed in the endometrium of the uterus.
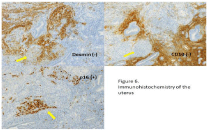
Figure 6: Immunohistochemistry of the excised uterus (×400). The tumor
cells invaded into the myometrium and were negative for desmin (arrow) and
CD10 (arrow), but positive for overexpression of p16.
Discussion
Mixed mesodermal tumors like the Mullerian adenosarcoma are usually classified in terms of being mixtures of benign or malignant tumor cells of both epithelial and mesenchymal origin. Adenofibroma and adenomyoma consist of benign epithelial cells and benign mesenchymal cells. Adenosarcoma consists of benign epithelial cells and malignant mesenchymal cells. Carcinofibroma consists of malignant epithelial cells and benign mesenchymal calls [17] (Table 2). Carcinosarcoma, consisting of epithelial and mesenchymal cells that are both malignant, are relatively common and are basically monoclonal in origin [18].
A Mullerian adenosarcoma of the endometrium occurring in an adolescent girl was first reported by Zaloudek in 1981 [2]. Prior to that, Craig et al., and Lipinski et al., had both reported finding a mixed mesodermal tumor arising in the corpus of a 14-year-old and 4-year-old, respectively [19,20]. However, these cases may have been carcinosarcomas. The tumor reported by Lipinski consisted of malignant epithelial cells and malignant mesenchymal cells. The case reported by Craig et al., advanced rapidly and the patient died within 4 months of her first surgery. Thesarcomatous component of Mullerian adenosarcomas of the uterus are typically homologous and low grade, this tumor type is generally considered to be less aggressive than its high-grade counterpart, or than carcinosarcoma [21]. Factors best associated with the worse prognosis of Mullerian adenosarcomas are reported to be advanced stage, extrauterine spread, myometrial invasion, the presence of Sarcomatous Overgrowth (SO), and Lymphovascular Space Invasion (LVSI) [14,22]. Ovarian metastasis of uterine adenosarcoma is reported to be rare, 2% [23].
Arend et al., reported that 84% of patients with stage IA disease and 51% of those with stage III disease survived for at least 5 years [24]. Carroll et al., suggested that advanced disease stage was associated with a worse Progression Free Survival (PFS) (stage II HR 3.14, 95% CI 1.41-6.98, p=0.005; stage III HR 24.70, 95% CI 4.95-123.40, p<0.001; stage IV HR 8.30, 95% CI 1.79-38.47, p=0.007) and that patients with earlier-stage disease had a longer time to first recurrence (median time, 28.1 months for stage I, 9.9 months for stage II, and 3.1 months for stage III, p=0.03) [12]. Extrauterine spread is reported to be a poor prognostic factor [25], as it leads the disease to stage III disease. Gallardo et al., suggested that the risk of recurrence in adenosarcoma with myometrial invasion was 36%, compared with 7% in cases without myometrial invasion [22].
The literature indicate that a preoperative radiological diagnosis of adenosarcoma is difficult. Tate et al., reported that a malignant tumor was correctly suggested in 63%, whereas a benign tumor was suspected in 12% of 67 adenosarcoma cases that had a preoperative radiological diagnosis [26]. Li et al., have described the preoperative diagnostic sensitivity for uterine sarcoma. The diagnostic sensitivity of ultrasound for uterine malignant tumors was 11%, much lower than for MRI (35%) and CT (63%) [27]. Ultrasound and hysteroscopy could detect only the presence of a polypoid lesion in some reports of adenosarcoma [12,28]. We assumed that a biopsy of the tumor would be needed for the proper diagnosis of an adenosarcoma although the MRI and CT could help in the prediction of myometrial invasion and extrauterine spread.
Early detection and treatment are frequently reported to improve the prognosis of mullerian adenosarcoma. However, there are reports suggesting that there are significant difficulties in its proper diagnosis, So some have tried repetitive hysteroscopic tumor biopsies. The most common difficulty with the diagnosis of Mullerian adenosarcoma is mostly because of the rarity of this tumor in young girls. Physicians would naturally assume that the abnormal vaginal bleeding of a young girl would be due to irregular menstruation. Additionally, it is difficult to perform a transvaginal examination for young girls. Adenofibroma, carcinosarcoma and Endometrial Stromal Sarcoma (ESS) can be a histological differential diagnosis, when a Mullerian adenosarcoma is suspected. We could have easily misdiagnosed our case if we had missed the malignant mesenchymal cells. In the case of carcinosarcoma, we could see not only malignant mesenchymal cells but also malignant epithelial cells. In the case of an ESS, the endometrial glands are not deformed by the proliferative stroma. A resection-biopsy, with a sufficient amount of tissue, is required for making this diagnosis. Immunohistochemistry could be an important in the diagnosis and detection of an adenosarcoma. In our case, it was difficult to recognize the tumor in the resected uterus, but negative CD10-staining and overexpression of p16 staining helped us to find the tumor in the myometrium.
There is currently no well-accepted guidelines for the treatment of Mullerian adenosarcoma of the endometrium for adolescent girls because in them the tumor is encountered so extremely rarely. The problem that looms the largest for the treatment of this disease in adolescent girls is the critical preservation of their fertility, if safely possible. For cases of Mullerian adenosarcoma of the cervix, several procedures have been reported that preserve fertility (conization, loop electrosurgical excision, and trachelectomy) [2,29,30]. But for cases of adenosarcoma of the endometrium, the procedure to preserve fertility is much more difficult.
To our knowledge, only 15 other such cases in under 20-yearolds have been reported, which are summarized, along with our new report, in (Table 1). Of these 16 cases, five patients initially received a local excision of the tumor, of which three later had recurrent disease. Two of the three-recuurent patients received hysterectomy and one received conization and dilation and curettage. Nine of the 16 cases immediately received a hysterectomy; two of them had recurrent disease and died soon thereafter. The procedure of local excision to preserve fertility may have a higher chance of disease recurrence, but the cases numbers and the amounts of associated metadata are too small to be certain.
It should be noted that, in case number 15 in (Table 1), L’Heveder et al., reported that an 18-year-old girl received similar conservative management, monitored every 6 months with pelvic ultrasound, hysteroscopy, and endometrial biopsy, with annual pelvic magnetic resonance imaging for 10 years. Eleven years later, she conceived following In-Vitro Fertilization (IVF) and successfully delivered. After 20 years of follow-up, she underwent a laparoscopic hysterectomy with no evidence of recurrence. The authors of that report proposed a management algorithm for uterine adenosarcoma and conservative management that could possibly be used for patients with stage IA tumors without myometrial invasion or SO. They proposed that hysteroscopic local excision of the tumor could be performed in cases where patients with stage IA uterine adenosarcoma, without MI or SO, desired to preserve their fertility-following full counseling and obtaining a second opinion.
Post-operatively, expert ultrasonography surveillance should be undertaken every 3 months for the first two years, every 6monthls until 5 years, and annually thereafter, in a similar fashion to other moderate to high-risk gynecological cancers. Hysteroscopy and endometrial biopsy should be applied if an abnormality is identified on ultrasound [16]. If our case had been an adenosarcoma of the endometrium, with a stage IA tumor without MI, we could have proposed a local excision. But it was very difficult for us to propose a conservative management plan because our case had detectable MI. A case series of 100 patients with uterine adenosarcoma reported by Clement included 4 patients treated with only a polypectomy and/or dilatation and curettage [6]. Zaloudek et al., reported 2 patients treated with only local resection in his case series [2]. All 16 patients in (Table 1) received some kind of surgery as their primary means of treatment. Adjuvant treatment after surgery is controversial for those patients who desire future fertility. Only case no.13, reported by Carroll et al., received adjuvant chemotherapy after a local resection. She went on to be disease-free for 132 months, although her history of pregnancy was unclear [14]. Adjuvant chemotherapy could be considered for young girls. Carroll et al., described 22 patients who received adjuvant chemotherapy and/or radiation therapy. They described the most common regimen of adjuvant chemotherapy as being, doxorubicin with ifosfamide [14]. Hormonal therapy could also be advocated. Uterine adenosarcoma showed 85% estrogen receptor positivity and 65% progesterone receptor positivity, however, the incidence of hormone receptor positivity is lower in patients with SO [30]. Carroll et al., reported on three patients with uterine adenosarcoma successfully treated with hormonal therapy [14]. Although data are limited, hormonal therapy could be considered for adjuvant therapy. Among patients with stage I disease with SO, adjuvant therapy trended to be associated with longer PFS and OS [14], however, the association was not statistically significant.
Genomic sequencing of adenosarcoma cases has been reported by Ban et al., they pointed out remarkable chromosome Copy-Number Variation (CNV) among their adenosarcoma cases [31,32]. They also determined that SO has a strongly association with HMGA2 overexpression, and with gene mutation of MAGEC1 and KDM6B. The aggressiveness of adenosarcoma might be judged with such genomic investigations. An accumulation of gene investigations of adenosarcoma cases is expected in the future.
In summation, early detection and correct identification of this rare tumor are of paramount importance if one is to consider any hope of fertility preservation. In our case, we decided to perform a TAH because a myometrial invasion was suspected by MRI before the surgery. The efficacy of our choice for ovarian preservation despite the uterine adenocarcinoma is still undetermined. We confirmed the benignity of the gross abnormalities on her ovaries and chose to preserve her ovaries for health and partial-fertility reasons. It is now possible for her to have a child with her eggs in a foster womb, if she so desires. The data regarding this rare tumor type is stillvery minimal, so it is equally difficult to define or recommend any reliable treatment or prognosis assessment. It is clear that further recognition and accumulation of data regarding Mullerian adenosarcoma of the endometrium in adolescent girls is needed, and studies of alternative treatments are required to establish an effective management strategy.
Acknowledgment
We would like to thank Dr. GS Buzard for his constructive critique and editing of our manuscript.
References
- Clement PB, Scully RE. Mullerian adenosarcoma of the uterus. A clinicopathologic analysis of ten cases of a distinctive type of Mullerian mixed tumor. Cancer. 1974; 34: 1138-1149.
- Zaloudek CJ, Norris HJ. Adenofibroma and adenosarcoma of the uterus: a clinicopathologic study of 35 cases. Cancer. 1981; 48: 354-366.
- Thornton JG. Mullerian adenosarcoma of the uterine corpus in a 14 year old girl. J Obstet Gynaecol 1986; 7: 76.
- Fujimori K, Yazaki S, Yamashita T. A case of Mullerian adenosarcoma. The Journal of Osaka Yamagata Prefecture Hospital (JPN) 1986; 20: 56-60.
- Gast MJ, Radkins LV, Jacobs AJ, Gersell D. Mullerian adenosarcoma of the cervix with heterologous elements: diagnostic and therapeutic approach. Gynecol Oncol. 1989; 32: 381-384.
- Clement PB, Scully RE. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of 100 cases with a review of the literature. Hum Pathol. 1990; 21: 363-381.
- Andrade LA, Derchain SF, Vial JS, Alvarenga M. Mullerian adenosarcoma of the uterus in adolescents. Int J Gynaecol Obstet. 1992; 38: 119-123.
- Fait T, Freitag P, Zivny J, Kuzel D. Adenosarcoma of the uterine body in a 19-year-old woman three year survival: Case report. Eur J Gynaec Oncol. 2001; 22: 61-63.
- Thiel DD, Erhard MJ. Uterine adenosarcoma in a boy with persistent Mullerian duct syndrome: first reported case. J Pediatr Surg. 2005; 40: e29-e31.
- Fatnassi R, Amri F. Adenosarcoma of the uterus: a case report. J Gynecol Obstet Biol Reprod. 2005; 34: 270-272.
- Okamoto K, Kimura H, Kawano M, Kamiyama M, Matsuzaki O. A case of uterine adenosarcoma in adolescents. Actuals of Obstet Gynecol (JPN). 2006; 55: 151-155.
- Ozmen B, Uzun N, Unlu C, Ortac F, Ataoglu O. Surgical conservation of both ovaries in an adolescent with uterine Mullerian adenosarcoma: a case report. J Minim Invasive Gynecol. 2007; 14: 375-378.
- Ozcan J, Dulger O, Kupelioglu L, Gonenc AI, Elsahin A. Uterine sarcoma in a 14-tear-old girl presenting with uterine rupture. Gynecol Oncol Rep. 2014; 10: 44-46.
- Carroll A, Ramirez PT, Westin SN, Soliman PT, Munsell MF, Nick AM, et al. Uterine adenosarcoma: an analysis on management, outcomes, and risk factors for recurrence. Gynecol Oncol. 2014; 135: 455-461.
- Hanyuan LB, Zhen SM, Dabao MS, Ying ZM. Uterine adenosarcoma with sarcomatous overgrowth: a case report of aggressive disease in a 16-yearold girl and a literature review. J Pediatr Adolesc Gynecol. 2018; 31: 426-431.
- L’Heveder A, Jones BP, Saso S, Barcroft J, Richardson R, Kaur B, et al. Conservative management of uterine adenosarcoma: lessons learned from 20 years of follow-up. Arc Gynecol Obstet. 2019; 300: 1383-1389.
- Tavassoli FA, Devilee P. Pathology and genetics of tumours of the breast and female genital organs. World Health Organization Classification of Tumours. 2003; 218: 247-249.
- Wada H, Enomoto T, Fujita M, Yoshino K, Nakashima R, Kurachi H, et al. Molecular evidence that most but not all carcinosarcomas of the uterus are combination tumors. Cancer Res. 1997; 57: 5379-5385.
- Craig JK. Inversion of the uterus associated with a malignant tumour in a girl of 14 years of age. J Obstet Gynaecol Br Emp. 1958; 65: 497-499.
- Lipinski A, Stulkowski K. Mixed mesodermal tumor of the uterine corpus in a 4-year-old girl. Ginekol Pol. 1974; 45: 881-885.
- Krivak TC, Seidman JD, McBroom JW, MacKoul PJ, Rose GS. Uterine adenosarcoma with sarcomatous overgrowth versus uterine carcinosarcoma: comparison of treatment and survival. Gynecol Oncol. 2001; 83: 89-94.
- Gallardo A, Prat J. Mullerian adenosarcoma: A clinic pathologic and immunohistochemical study of 55 cases challenging the existence of adenofibroma. Am J Surg Pathol. 2009; 33: 278-288.
- Arend R, Bagaria M, Lewin SN, Sun X, Deutsch I, Burke WM, et al. Longterm outcome and natural history of uterine adenosarcomas. Gynecol Oncol. 2010; 119: 305-308.
- Kaku T, Silverberg SG, Major FJ, Miller A, Fetter B, Brady MF. Adenosarcoma of the uterus: a gynecologic oncology group clinicopathologic study of 31 cases. Int J Gynecol Pathol. 1992; 11: 75-88.
- Tate K, Watanabe R, Yoshida H, Shimizu H, Uehara T, Ishikawa M, et al. Uterine adenosarcoma in Japan: Clinicopathologic features, diagnosis and management. Asia-Pac J Clin Oncol. 2018; 14: 318-325.
- Li D, Yin N, Du G, Wang S, Xiao Z, Chen J, et al. A real-world study on diagnosis and treatment of uterine sarcoma in western China. Int J Biol Sci. 2020; 16: 388-395.
- Fleming NA, Hopkins L, Nanassy J, Senterman M, Black AY. Mullerian adenocarcinoma of the cervix in a 10-year-old girl: case report and review of the literature. J Pediatr Adolesc Gynecol. 2009; 22: e45-e51.
- Dubuc E, Gascon S. Four-year remission after a conservative treatment in a teenager with a low-grade adenosarcoma of the cervix. J Pediatr Adolesc Gynecol. 2010; 23: e79-e80.
- Shinnick JK, Kumer N, Beffa L, Miller K, Friedman MA, Kalife E, et al. Management of low-grade cervical Mullerian adenosarcoma in a 14-year-old girl. J Pediatr Adolesc Gynecol. 2017; 30: 652-654.
- Amant F, Schurmans K, Steenkiste E, Verbist L, Abeler VM, Tulunay G, et al. Immunohistochemical determination of estrogen and progesterone receptor positivity in uterine adenosarcoma. Gynecol Oncol. 2004; 93: 680-685.
- Ban Y, Fischer JV, Maniar KP, Guo H, Zeng C, Li Y, et al. Whole-Genome Sequencing and Target Validation Analysis of Müllerian Adenosarcoma: A Tumor with Complex but Specific Genetic Alterations. Front Oncol. 2020; 10: 538.
- Michener CM, Simon NL. Ovarian conservation in a woman of reproductive age with Mullerian adenosarcoma. Gynecol Oncol. 2001; 83: 424-427.
