Abstract
We present a case of patient with spontaneous cardiac tamponade related to a very high 2-plasma concentration of dabigatran, an oral direct-acting thrombin inhibitor. By selectively inhibiting thrombin alone, dabigatran may have antithrombotic efficacy while preserving some other hemostatic mechanisms in the coagulation system and thus potentially mitigating the risk of bleeding. Nonetheless, serious bleeding can occur with dabigatran. We illustrate the management of this life threatening hemorrhagic complication by the combination of cardiac surgery, antagonization of the anticoagulant effect (using Idarucizumab, an humanized monoclonal antibody fragment and continuous renal replacement therapy), and monitoring of the effects on coagulation by thromboelastogram.
Introduction
Dabigatran is a new oral direct-acting thrombin inhibitor effective in the prevention of Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE) and stroke in patients with Atrial Fibrillation (AF). Dabigatran has interesting pharmacokinetic and pharmacodynamic properties: non-vitamin dependent, metabolism independent of liver function, and with a few drug interferences [1]. By selectively inhibiting thrombin alone, dabigatran may have antithrombotic efficacy while preserving some other hemostatic mechanisms in the coagulation system and thus potentially mitigating the risk of bleeding. These properties associated with the uselessness of biological monitoring explain the success of new oral anticoagulants.
Compared to warfarin, treatment with dabigatran (150 mg twice daily) for AF and for the treatment of PE and DVT was associated with a higher rate of gastrointestinal bleeding and a lower rate of intracranial bleeding [1-3]. Nonetheless, serious bleeding can occur with dabigatran. Dabigatran is mainly (80%) excreted by the kidneys (its serum half-life is between 12 and 17 hours), making its use more limited in cases of renal failure [1]. In case of severe and active bleeding, the anticoagulant effect cannot be reversed with vitamin K, unlike warfarin. Idarucizumab is a humanized monoclonal antibody fragment that has been developed as a specific reversal agent for dabigatran. Idarucizumab selectively and exclusively binds dabigatran with an affinity ≈350-fold higher than the affinity of dabigatran for thrombin and neutralizes its anticoagulant effect immediately, especially in case of uncontrollable bleeding [4]. We present a case of an 84-year-old patient with spontaneous tamponade in a context of high plasma concentration of dabigatran, who required idarucizumab, four-factor prothrombin complex concentrates and continuous renal replacement therapy to control this life-threatening situation.
Case Presentation
An 84-year-old male with history of hypertension, AF, hyperlipidemia, severe arteriopathy, usually taking dabigatran (150mg twice daily), ACE-inhibitor with hydrochlorothiazide, and ezetimibe, presented to the emergency department for asthenia and dyspnea worsening since several days. Physical examination and vital parameters showed poor skin perfusion, lethargy, jugular turgor, tachycardia with paradoxical pulse, and arterial hypotension. Cardiac tamponade characterized by a circumferential pericardial effusion with compression of the right cavities was diagnosed by the use of cardiac ultrasound. Admission blood tests showed lactic acidosis (3.6mEq/L), acute renal failure KDIGO I (creatinine: 208.5μmol/L, uremia: 39.2mmol/L) and a significant alteration of standard coagulation tests: Activated Partial Thromboplastin Time (APTT) at 127s (normal range from 30 to 40 s) and Prothrombin Time (PT) at 143s (normal range from 11 to 13, 5s). Fibrinogen concentration and platelets count were in the normal ranges. The plasma concentration of dabigatran at admission was 368ng/mL. The patient reported no medication error.
To allow a sub-xyphoid pericardial puncture in order to evacuate the pericardial effusion, a reversion of dabigatran was quickly started by the administration of 50U/Kg of four-factor prothrombin complex concentrates (COFACT®) and with a 5g dose of idarucizumab. 200 cc of bloody liquid were drained under ultrasound guidance, resulting in a rapid resolution of the shock. However, no drain could be left in place due to an excessively thick pericardium. Thereafter, cardiac ultrasound examinations showed a very gradual recurrence of pericardial without compression of cavities. Despite the administration of a first dose of idarucizumab, the plasma level of dabigatran was still higher than the initial concentration (490ng/mL). Standard coagulation tests Remained Altered and Thromboelastogram (ROTEM®) showed a significant extended clotting time. Renal function deteriorated to a grade KDIGO III (creatinine: 586.9μmol/L, uremia 91.7mmol/L). In this context, Renal Replacement Therapy (RRT) by continuous venovenous hemodiafiltration (blood flow: 180ml/min; dialysate flow: 2000ml/h; no fluid withdrawal) was started in order to increase the clearance of dabigatran. In the following hours, successive biological analyzes showed a significant decrease in the plasma concentration of dabigatran as well as a progressive resolution of the coagulopathy on all tests (Figure 1 and Table 1).
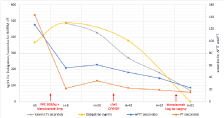
Figure 1: Evolution of coagulation parameters since admission. PPC: fourfactor
prothrombin complex concentrates.
CT: Clotting Time on Thromboelastogram; CVVHDF: Continuous Veno-
Venous Hemodiafiltration; APTT: Activated Partial Thromboplastin Time; PT:
Prothrombin Time.
APTT
(second es)PT
(second es)Extern CT
(second es)Dabigatran
(ng/ml)HO
127
143
368
H+8
556
217
486
490
H+30
61
344
427
H+40
483
224
272
378
H+50
388
192
179
H+52
228
154
68
<20
CT: Clotting Time on Thromboelastogram; APTT: Activated Partial Thromboplastin Time; PT: Prothrombin Time.
Table 1: Evolution of coagulation parameters since admission.
Nevertheless, on day 3, we observed a recurrence of cardiac tamponade that required surgical pericardial drainage 20 hours after the initiation of RRT. A second 5g dose of idarucizumab was administered before the incision in order to perform the surgery in the best coagulation conditions. The surgery was done without difficulty in hemostasis. Dabigatran plasma concentration was less than 20ng/ mL and APTT, PT and ROTEM® were in normal range. The patient no longer suffered from bleeding complications and was able to fully recover from his heart and kidney failure, without further dialysis. Finally, anatomopathological analyzes of the pericardial liquid identified a lymphoma.
Discussion
This clinical case illustrates the complexity of managing lifethreatening bleeding in a patient with high plasma concentration of dabigatran. In a prespecified analysis of the RE-LY study, the twice daily 150mg dose of dabigatran (taken by our patient) was associated with a more than 5-fold variation of plasma concentration, indicating a wide therapeutic range (median peak plasma concentrations of 184 ng/ml [10th-90th percentile 74.3-383 ng/mL) [4]. The risks of major bleeding after dosing 150mg dabigatran in patients with AF were related to trough concentrations of dabigatran [4]. As our patient did not make a mistake in taking his medication, it seems likely that other factors could have influenced his results. Indeed, significant factors influence dabigatran plasma concentrations such as age, renal function, and gender [4]. Indeed, there is a great variability in the blood concentration of dabigatran in the geriatric population, and levels above ≥243.9 ng/mL seem to be associated with a risk of bleeding [5].
Interestingly, we observed an increase in the plasma concentration of dabigatran during the first hours of hospitalization. The most likely explanation for a recurrent increase in dabigatran level, which was seen at 12 h from the admission, is redistribution of unbound dabigatran from the extravascular to the intravascular compartment [6].
Idarucizumab, a humanized monoclonal antibody fragment, has been developed for a specific reversal agent for dabigatran. Idarucizumab binds dabigatran with a high affinity and once dabigatran is complexed to idarucizumab, the anticoagulant effects of unbound and proteinbound dabigatran and its active metabolites are neutralized. In vitro and ex vivo studies have demonstrated that idarucizumab promptly restores dabigatran-prolonged coagulation parameters to baseline values [7]. In 503 patients who were receiving dabigatran, the REVERSE AD trial showed 5g idarucizumab reversed anticoagulation rapidly in more than 98% of the patients [8]. However, a single dose of 5g idarucizumab may not be sufficient to reverse the anticoagulant effects of dabigatran especially during circumstances favoring significant high concentration such as renal failure [9,10].
About 35% of dabigatran is bound to plasma protein and >80% of the drug is renally cleared; the rest is eliminated through biliary excretion [11]. The utility of RRT as a mechanism for expedited elimination of dabigatran may be an option for patients undergoing emergency surgery or in the setting of life-threatening bleeding [12,13]. Conventional dialysis is more effective, but rebounded up seem more important than with CVVH [14,15]. The extracorporeal therapy reduced the plasma concentration of dabigatran by more than 50% in 24 hours. The combination of RRT by CVVH and a second dose of 5g idarucizumab made it possible to perform the surgical intervention in good hemostasis conditions. It can be difficult to evaluate activity of dabigatran by a common coagulant test, such as INR or APTT, because may be within their normal ranges although important concentration [16]. ROTEM Clotting Time (CT) appears to be well correlated with circulating dabigatran, with the advantage of being a point-of-care and rapid response [17,18].
Conclusion
This clinical case illustrates the complexity of managing lifethreatening bleeding in a patient with very high plasma concentration of dabigatran and highlights the importance of monitoring the reversal effect of idarucizumab. A single dose may not be sufficient. Treatment should therefore be individualized.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N Engl J Med. 2009; 361: 1139-1151.
- Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus Warfarin in the Treatment of Acute Venous Thromboembolism. N Engl J Med. 2009; 361: 2342-2352.
- Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014; 129: 764-772.
- Eikelboom JW, van Ryn J, Reilly P, Hylek EM, Elsaesser A, Glund S, et al. Dabigatran Reversal with Idarucizumab in Patients With Renal Impairment. J Am Coll Cardiol. 2019; 74: 1760-1768.
- Chaussade E, Hanon O, Boully C, Labouree F, Caillard L, Gerotziafas G, et al. Real-Life Peak and Trough Dabigatran Plasma Measurements Over Time in Hospitalized Geriatric Patients with Atrial Fibrillation. J Nutr Health Aging. 2018; 22: 165-173.
- Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008; 47: 285-295.
- Schiele F, van Ryn J, Canada K, Newsome C, Sepulveda E, Park J, et al. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013; 121: 3554-3562.
- Pollack CV, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, et al. Idarucizumab for Dabigatran Reversal. N Engl J Med. 2015; 373: 511-520.
- Steele AP, Lee JA, Dager WE. Incomplete dabigatran reversal with idarucizumab. Clin Toxicol. 2018; 56: 216-218.
- Rottenstreich A, Jahshan N, Avraham L, Kalish Y. Idarucizumab for dabigatran reversal-Does one dose fit all? Thromb Res. 2016; 146: 103-104.
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos Biol Fate Chem. 2008; 36: 386-399.
- Chai-Adisaksopha C, Hillis C, Lim W, Boonyawat K, Moffat K, Crowther M. Hemodialysis for the treatment of dabigatran-associated bleeding: a case report and systematic review. J Thromb Haemost. 2015; 13: 1790-1798.
- Chang DN, Dager WE, Chin AI. Removal of Dabigatran by Hemodialysis. Am J Kidney Dis. 2013; 61: 487-489.
- Chiew AL, Khamoudes D, Chan BSH. Use of continuous veno-venous haemodiafiltration therapy in dabigatran overdose. Clin Toxicol. 2014; 52: 283-287.
- Singh T, Maw TT, Henry BL, Pastor-Soler NM, Unruh ML, Hallows KR, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol CJASN. 2013; 8: 1533-1539.
- Cuker A, Siegal DM, Crowther MA, Garcia DA. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol. 2014; 64: 1128-1139.
- Henskens YMC, Gulpen AJW, van Oerle R, Wetzels R, Verhezen P, Spronk H, et al. Detecting clinically 160 relevant rivaroxaban or dabigatran levels by routine coagulation tests or thromboelastography in a cohort of patients with atrial fibrillation. Thromb J. 2018; 16: 3.
- Taune V, Wallen H, Agren A, Gryfelt G, Sjovik C, Wintler AM, et al. Whole blood coagulation assays ROTEM and T-TAS to monitor dabigatran treatment. Thromb Res. 2017; 153: 76-82.
