Abstract
Group G Streptococcus (GGS) causes toxic shock syndrome. Its incidence has been increasing in the elderly in recent years.
The case is a female patient in her 60s, with rheumatoid arthritis, who developed necrotizing fasciitis in her right thigh. We administered antibacterial agents and debridement frequently at an early stage, followed by aggressive high-protein enteral nutrition and multidisciplinary treatment. During the course of treatment, the patient’s general condition temporarily deteriorated because of fecal contamination. We considered amputation of the lower limb and implantation of a stoma, but finally succeeded in preserving the limb by performing two skin grafts. The patient was able to walk and was discharged on day 66.
GGS infection is a risk factor for elderly patients with underlying diseases such as malignancy or immunocompromised states. When necrotizing fasciitis due to fulminant streptococcal infection develops in a proximal limb, amputation of the limb is often necessary to save the patient’s life. In this case, a fulminant GGS infection developed in an immunocompromised patient with active rheumatoid arthritis. Although the risk of limb amputation was high, multidisciplinary treatment enabled functional preservation of the affected limb.
Keywords: GGS; STSS; Necrotizing fasciitis; Fulminant streptococcal infections
Abbreviations
GGS: Group G Streptococcus; GAS: Group A Streptococcus; qSOFA: quick Sequential Organ Failure Assessment; ICU: Intensive Care Unit; CHDF: Continuous Hemodiafiltration
Introduction
Necrotizing fasciitis is a rapid-spreading, necrotizing soft tissue infection, with the shallow fascia as the main site of bacterial infection. GGS derives from the normal bacterial flora of the human upper respiratory tract, and is endemic to the skin, gastrointestinal tract, and female genital tract. It has been implicated in endocarditis, septic arthritis, osteomyelitis, fetal infections, and meningitis.
GGS has genomic homology with Group A Streptococcus (GAS). Like GAS, it is thought to cause streptococcal toxic shock syndrome. Currently, GGS infections are attracting attention because of the increasing number of elderly people affected. Necrotizing fasciitis caused by GGS requires early therapeutic intervention. However, there are few reports on the prognosis of necrotizing fasciitis caused by GGS alone or of the amputation rate of affected limbs.
Case Presentation
The patient is a female in her 60s. She had been taking methotrexate, tocilizumab, and iguratimod for the treatment of rheumatoid arthritis. One day, she scraped her right heel and later visited a dermatologist. She was treated for cellulitis but became feverish, had swelling of the right thigh and difficulty walking; she was admitted to a nearby hospital. Subsequently, the swelling worsened and extensive redness and blood blisters appeared. She was suspected of having necrotizing fasciitis and was referred to our hospital for further evaluation and treatment.
On admission, there was no disturbance of consciousness, body temperature was 37.50C, heart rate was sinus tachycardia 124 bpm, blood pressure was 84/40 mmHg, respiratory rate was 30 tachypneas, and the quick Sequential Organ Failure Assessment (qSOFA) score was 2 points. Physical examination of the chest and abdomen showed no abnormal findings. However, there was spontaneous pain and tenderness on the right thigh, accompanied by redness, swelling, and blister formation. In addition, a crusting with exudate was observed on the right heel (Figure 1).
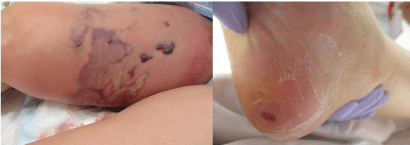
Figure 1: Physical examination. Skin findings on the medial side of the right thigh. Erythema, swelling, and blister formation were noted. Crusting with exudate
was observed on the right heel.
In blood tests, we found metabolic acidosis and elevated procalcitonin. We suspected multiple organ failure due to sepsis. Acute Kidney Injury (AKI) was 9 points of SOFA score and 4 points of Laboratory Risk Indicator for Necrotizing Fasciitis score (Figure 2).
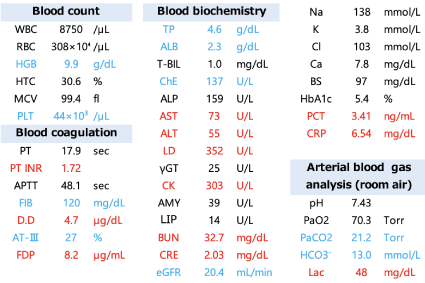
Figure 2: Blood test.
We diagnosed the patient as having necrotizing fasciitis of the right thigh with septic shock. We admitted the patient to the Intensive Care Unit (ICU) and began treatment with several different antibiotics; crystalloids, inotropes, and Continuous Hemodiafiltration (CHDF) were administered for AKI. We also started debridement on the day of admission. The clinical course is shown in Figure 3.
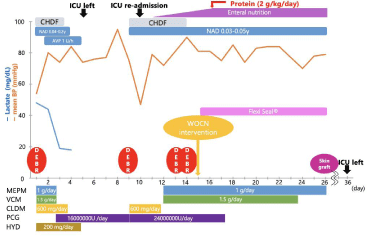
Figure 3: Clinical course. MEPM: Meropenem, VCM: Vancomycin, CLDM: Clindamycin, PCG: Penicillin G
HYD: Hydrocortisone, CHDF: Continuous Hemodiafiltration, NAD: Noradrenaline
AVP: Vasopressin, DEBR: Debridement.
On the second day, blood culture showed gram-positive streptococcus. The antibiotics were de-escalated to Penicillin G. Thereafter, daily incision and washout procedures were performed; however, gradually progressive necrosis was observed on the medial and posterior sides of the right thigh. On the 12th day, respiratory depression and hypotension progressed; the patient was again admitted to the ICU and managed with a ventilator.
Although tube feeding had to be interrupted due to deterioration of the patient’s general condition, it was resumed with a target of 2 g/ kg/day of protein, taking into account loss of protein due to wound healing and loss of protein and amino acids due to CHDF. On the 16th day, the administration of target calories (25 kcal/kg/day) and target protein was achieved.
Initially, we considered adding a stoma or amputating the lower limbs to prevent wound infection due to fecal contamination. However, the nurse in wound, ostomy, and continence intervened and inserted a Flexi-Seal® rectal tube (ConvaTec, Reading, UK). This procedure improved the wound infection caused by fecal contamination and prevented amputation of the lower limb. With frequent washing, debridement, and appropriate enteral nutrition, the necrotic spread of the wound was eliminated, and good granulation was observed. The first skin transplant procedure was performed on day 26. She was discharged from the ICU on day 36, and the second skin grafting procedure was performed on day 41. Thereafter, the epithelialization of the skin improved steadily, she was able to walk on her own, and was discharged home on day 66 (Figure 4).
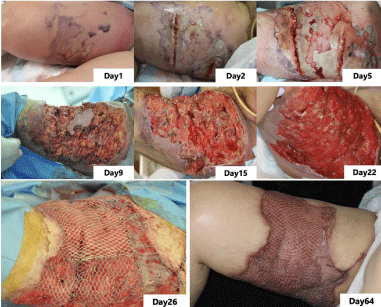
Figure 4: Progress of skin findings. Debridement was enforced almost daily. Skin transplant procedure was performed on day 26 and the day 41. The patient was
discharged on the day 66.
Discussion
Most of the causative organisms of necrotizing fasciitis are GASs, and GGSs have not received much attention, however recently, GGSs, which have been considered nonpathogenic, have been recognized as important bacterial pathogens [1,2]. In particular, GGS infection has been reported to cause symptoms similar to GAS-induced fascial necrosis and streptococcal shock-like syndrome in adults with underlying diseases [3,4].
GAS shows bimodal, age-specific peaks in the 0-2 and 70- 79 year age groups, whereas GGS is found in the 70-79 age group. For this reason, GGS has been attracting attention in recent years, as the number of elderly people increases. Furthermore, the risk of GGS infection has been reported to be associated with older age, cardiovascular disease, diabetes, liver cirrhosis, alcoholism, bone and joint disease, skin disease, immunocompromised conditions, and post-surgery [3,5].
This patient was using multiple immunosuppressive drugs for rheumatoid arthritis, which may have lowered her immunity and increased her risk of GGS infection.
The prognosis and lower limb amputation rates for necrotizing fasciitis due to GGS infection have been studied in only a few singlecenter studies.
Necrotizing fasciitis of the lower leg has been reported to be most commonly caused by GAS, followed by GGS. In necrotizing fasciitis of the extremities, older age and higher lactate levels are associated with significantly higher 90-day mortality rates. In this case, lactate levels (48 mg/dL) were high, and 90-day mortality risk was considered high.
On the other hand, debridement more than three times has been reported to improve the prognosis [6,7], and in this case, frequent attempts at debridement may have reduced the risk of amputation of the affected limb and death.
Regarding nutrition in sepsis and septic shock, it is recommended in Japan to aim for less than 1.0 g/kg/day of protein in the acute phase, but the European Society for Clinical Nutrition and Metabolism guidelines recommend a target of 1.3 g/kg/day of protein in ICU patients, because of the high amount of protein consumed and degraded by catabolism [8,9]. Since amino acid loss in CHDF is estimated to be 10-15 g/day of nutritional disturbance, the protein dose should be increased after the introduction of hemodialysis, to account for the amount of protein lost by hemodialysis. Therefore, it is necessary to increase the protein dose during CHDF, and the American Society for Parenteral and Enteral Nutrition/Society of Critical Care Medicine (ASPEN/SCCM) guidelines suggest a maximum dose of 2.5 g/kg/day, with an additional dose of 0.5 g/kg/ day [10,11].
In this case, careful debridement was performed for necrotizing fasciitis. The target protein level was set at 2 g/kg/day, taking into account the amount of protein used for wound healing and the amount of protein lost during CHDF. This may have contributed to infection control, good granulation, and wound healing. Fortunately, she was able to walk and leave the hospital.
Conclusion
GGS infections, like GAS infections, make necrotizing fasciitis more likely to be severe and carry the risk of lower extremity amputation. We believe that multidisciplinary treatment, including early aggressive debridement, appropriate high-protein nutrition, and wound infection control is important for the treatment of severe necrotizing fasciitis and preservation of the affected limb.
References
- Ikebe T, Murayama S, Saitoh K, Yamai S, Suzuki R, Isobe J, et al. Surveillance of severe invasive group-G streptococcal infections and molecular typing of the isolates in Japan. Epidemiol Infect. 2004; 132: 145-149.
- Brandt CM, Spellerberg B. Human infections due to Streptococcus dysgalactiae subspecies equisimilis. Clin Infect Dis. 2009; 49: 766-772.
- Takahashi T, Sunaoshi K, Sunakawa K, Fujishima S, Watanabe H, Ubukata K. Clinical aspects of invasive infections with Streptococcus dysgalactiae ssp. equisimilis in Japan: differences with respect to Streptococcus pyogenes and Streptococcus agalactiae infections. Clin Microbiol Infect. 2010; 16: 1097- 1103.
- Shimomura Y, Okumura K, Murayama SY, Yagi J, Ubukata K, Kirikae T, et al. Complete genome sequencing and analysis of a Lancefield group G Streptococcus dysgalactiae subsp. equisimilis strain causing streptococcal toxic shock syndrome (STSS). BMC Genomics. 2011; 12: 17.
- Ubukata K, Sunaoshi K, Kobayashi R, Okuzumi K. Large-scale Questionnaire Surveillance Concerning Invasive Infections with Group C and G Streptococci. The Journal of the Japanese Association for Infectious Diseases. 2006; 80: 480-487.
- Madsen MB, Skrede S, Perner A, Arnell P, Nekludov M, Brunn T, et al. Patient’s characteristics and outcomes in necrotising soft-tissue infections: results from a Scandinavian, multicentre, prospective cohort study. Intensive Care Med. 2019; 45: 1241-1251.
- Bruun T, Kittang BR, de Hoog BJ, Aardal S, Flaatten HK, Langeland N, et al. Necrotizing soft tissue infections caused by Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis of groups C and G in western Norway. Clin Microbiol Infect. 2013; 19: E545-E550.
- The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock. 2020; J-SSCG 2020: E247-E264.
- Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019; 38: 48-79.
- McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN.). JPEN J Parenter Enteral Nutr. 2016; 40: 159-211.
- Martindale RG, McClave SA, Vanek VW, McCathy M, Roberts P, Taylor B, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med. 2009; 3: 1757-1761.
