Abstract
Liver impairment is frequently reported in Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) infected patients and contributes to increased morbidity and mortality in critically ill Coronavirus disease-2019 (COVID-19) patients. We report of a 44-year-old male patient with hypoxic and cholestatic liver failure after an initially complicated course of COVID-19 pneumonia with moderate Acute Respiratory Distress Syndrome (ARDS), Acute Kidney Injury (AKI) stage 3 with Kidney Replacement Therapy (KRT), thromboembolic intestinal ischemia with subtotal colectomy and partial resection of the small intestine and septic shock. After considerable clinical improvement we initiated extracorporeal liver support due to progressive hyperbilirubinemia up to 25,3 mg/dl.
Within 17 days we conducted 11 sessions of extracorporeal liver support by Fractionated Plasma Separation and Adsorption (FPSA; Prometheus®) until stabilization of liver function occurred. After 52 days of intensive care treatment and successful weaning from ventilation and KRT, the patient was transferred to an Intermediate Care (IMC) unit.
To the best of our knowledge, this is the first report of a COVID-19 patient successfully treated with prolonged extracorporeal liver support. Extracorporeal procedures that support liver function should be considered as bridging to recovery in selected COVID-19 patients if liver failure presents a dominant organ dysfunction.
Keywords: COVID-19; Liver failure; Extracorporeal liver support; Critical care
Abbreviations
AKI: Acute Kidney Injury; ALF: Acute Liver Failure; ARDS: Acute Respiratory Distress Syndrome; COVID-19: Coronavirus Disease-2019; CRP: C-Reactive Protein; CT: Computertomography; ECMO: Extracorporeal Membrane Oxygenation; FPSA: Fractionated Plasma Separation and Adsorption; ICU: Intensive Care Unit; IMC: Intermediate Care Unit; KRT: Kidney Replacement Therapy; MARS: Molecular Adsorbent Recirculating System; MELD: Model of End Stage Liver Disease; MODS: Multiple Organ Dysfunction Syndrome; PCR: Polymerase Chain Reaction; PCT: Procalcitonin; SARS-CoV-2: Severe Acute Respiratory Syndrome- Coronavirus- 2; SOFA Score: sepsis Related Organ Failure Assessment Score; SPAD: Single- Pass Albumin Dialysis; WBCC: White Blood Cell Count
Background
Emerging evidence shows that COVID-19 is a multi-organ disease that can directly or indirectly induce varying degrees of liver dysfunction. Liver dysfunction has been repeatedly described in COVID-19 patients – but in most cases the degree of hepatic injury is mild and transient [1-5]. However, in critically ill COVID-19 patients liver dysfunction correlates with morbidity and mortality and liver function has been proposed to be a marker of disease progression [2,4,6,7]. Direct virus infection of hepatocytes and cholangiocytes, imbalanced immune responses, coagulation abnormalities, systemic inflammation, ischemia, hypoxia, hepatic congestion, and drug toxicity are possible mechanisms for hepatic dysfunction in COVID-19 patients [2,3,6-8].
Knowledge about extracorporeal lung support in critically ill COVID-19 patients is emerging, whereas data on extracorporeal liver support in COVID-19 patients is limited [9]. Here we report a COVID-19 patient with severe ischemic cholangiopathy successfully treated with the extracorporeal liver support system Prometheus® (Fresenius Medical Care, Bad Homburg, Germany) in terms of bridging to recovery.
Case Presentation
The 44-year-old male patient without comorbidities was admitted to hospital with fever, cough, thoracic pain, and myalgia due to SARSCoV- 2 infection. Within the next 12 days the pulmonary status deteriorated progressively, necessitating transfer to our academic referral hospital for consideration of extracorporeal membrane oxygenation (ECMO) support on a specialized ARDS ICU (intensive care unit).
On admission to our hospital the patient presented with moderate ARDS, concomitant AKI requiring KRT and a sepsis related organ failure assessment score (SOFA) of 17. Radiologic work-up showed large patchy consolidations in line with COVID-19 pneumonia but no other pathologic findings.
The laboratory on admission showed a normal serum bilirubin concentration (0.5 mg/dl) and mildly elevated liver enzymes (AST: 143 U/l, ALT: 82 U/l, GGT 60 U/l, AP: 44U/l, LDH: 527 U/l, INR: 1,09). The Model of End Stage Liver Disease Score (MELD) on admission was 21. A viral hepatitis was excluded in the standardized ARDS work-up at admission. A pulmonary bacterial co-infection was assumed because of elevated concentrations of standard inflammation biomarkers (white blood cell count (WBCC) 11/nl, C-Reactive Protein (CRP) 29.7 mg/dl, Procalcitonin (PCT) 1.8 ng/ ml) – but microbiologic work-up remained negative and the initial antibiotic therapy with piperacillin/ tazobactam and clarithromycin was stopped after 9 days and 6 days, respectively. As part of standardized therapy of COVID-19 by that time hydroxychloroquine was also given for 10 days.
Pulmonary function over the next days improved markedly due to optimized lung protective invasive ventilation and intermittent prone positioning. Vasopressor support was completely weaned, ventilatory support was gradually reduced and inflammatory biomarkers returned to normal values. Repeated spontaneous awakening trials with complete sedation interruption showed no neurologic arousal. Computertomographic (CT) diagnostic of the head showed no pathology but further work-up detected severe hyperammonia (457 μg/dl).
Measures to reduce ammonia levels were initiated (such as oral rifaximin, oral ursodeoxycholic acid, oral lactulose, and intravenous l- ornithine aspartat), resulting in a decline of ammonia levels. However, bilirubin levels showed a steady increase up to 16,8mg/ dl, resulting in a MELD score of 32 (Figure 1). The following day the patient developed acute thromboembolic mesenteric ischemia with consecutive septic shock necessitating emergency surgery. A subtotal colectomy and partial resection of the small intestine was performed with creation of a terminal ileostoma. Intraoperative liver inspection showed a severely discoloured liver, and a liver biopsy was performed. The patient’s clinical status stabilized within 36 hours following surgical focus control and standardized sepsis therapy. Hemodynamic and pulmonary function recovered completely, but severe liver dysfunction persisted (MELD: 33; SOFA: 16).
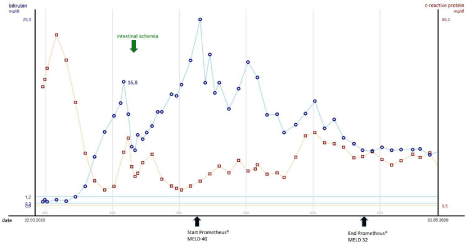
Figure 1: Time course of serum bilirubin-, c- reactive protein-levels and MELD score during the patient’s ICU stay showing start and end of extracorporeal liver
support (MELD: model of endstage liver disease).
Seven days after the emergency surgery, liver failure (MELD score 40) remained the dominant organ dysfunction due to prominent hyperbilirubinemia and hyperammonaemia (Figure 1).
The neurologic situation remained inconclusive without adequate awakening. A follow-up head CT- scan remained without any pathology. However, the pulmonary, hemodynamic, and inflammatory situation improved constantly. Liver transplantation evaluation was discarded due to persisting SARS-CoV-2 infection and multiorgan failure. The liver biopsy showed liver cell necroses in about 80% of the examined area and cholestasis with bile infarction and degenerative inflammatory changes in the bile ducts. Immunohistochemical staining showed a beginning chronic cholestasis. SARS-CoV-2 tissue PCR (polymerase chain reaction) or immunohistochemistry were not available at the time of biopsy.
After interdisciplinary discussion and information of the patient’s relatives regarding therapeutic options, FPSA was started as trial for bridge to recovery therapy. The Prometheus® therapy was hemodynamically well tolerated by the patient. Effective treatment duration of each FPSA-session was 6 hours, anticoagulation was performed using citrate, blood flow rate was 200 ml/min, plasma flow rate 300 ml/min and dialysate flow rate 300 ml/min. Bilirubin- and ammonia levels were effectively reduced, and the patient showed an adequate neurologic arousal within 5 days. After 9 cycles on Prometheus® therapy he was neurologically adequate. Liver function recovered (Figure 1) and Prometheus® therapy was terminated after 11 treatment cycles (MELD score 32). Complete weaning from ventilation and tracheostomy was feasible in the neurologic adequate patient. Kidney function recovered permitting intermittent KRT. After 52 days the neurologically adequate patient was transferred to an IMC to continue treatment for critical illness polyneuropathy and myopathy. Of note, the first negative SARS-CoV-2 test (PCR from bronchoalveolar-lavage sample) result was observed 5 days following the start of Prometheus® therapy.
Discussion
Liver dysfunction has been demonstrated to be a key clinical characteristic in critically ill COVID-19 patients, but fulminant liver failure seems to be rare [2,3,6]. Severe liver failure occurs in up to onethird of critically ill COVID-19 patients and is associated with high mortality [7,10]. Previous case reports with COVID-19 associated Acute Liver Failure (ALF) showed comparable clinical courses to our report underlining the multifactorial causality for liver dysfunction in SARS-CoV-2 infected critical ill patients [7,8,11-14]. Precise recommendations on the effective timing of artificial liver support or the optimal extracorporeal technology to treat different aetiologies of liver dysfunction are not established. Data on extracorporeal liver support in COVID-19 are limited but one aspect that is currently discussed based on recent findings is extracorporeal elimination of toxins and cytokines to attenuate adverse effects of cytokine storm associated with severe SARS-CoV-2 infection [9,15].
The FPSA separates plasma with molecules up to the size of 68 kDa into a second circuit, where albumin-bound toxins are directly adsorbed by a neutral resin adsorber (Prometh® 01) and an anion exchanger (Prometh® 02). Afterwards, this purified plasma returns to the primary circuit, which passes the whole blood through a highflux hemodialyzer to remove water-soluble substances by diffusion [16,17]. The efficiency of the Prometheus® system for detoxification in patients with liver failure has been established-including inflammatory cytokine removal [16,17]. Previous results indicated survival benefits for Prometheus treatment in patients with severe liver disease (MELD >30) and with hepatic encephalopathy [17,18].
In this COVID-19 patient use of the Prometheus® system proved to be beneficial in the clinical management to ascertain neurologic function and to guide treatment decisions. Criteria considered before extracorporeal liver support included the physical status before COVID-19, recovery of pulmonary and hemodynamic status following emergency surgery and the progressive hyperbilirubinemia with a MELD score of 40. The bridging strategy proved to be effective given the parallel clinical recovery following COVID-19 associated ARDS and MODS (multiple organ dysfunction syndrome). It remains an intriguing idea that extracorporeal liver support could have had positive effects on cytokine toxicity in this patient. A study of 12 critical ill COVID-19 patients treated with an artificialliver blood-purification system showed a significantly drop of 32 analysed cytokines and underlines this hypothesis [15]. We concede other methods of artificial liver support might also be successful in such instances for example the Molecular Adsorbent Recirculating System™ (MARS™), high plasma volume exchange or Single-Pass Albumin Dialysis (SPAD).
Conclusion
Extracorporeal liver support such as the Prometheus® system should be considered as bridge to recovery therapy in selected COVID-19 patients with ALF.
References
- Bertolini A, van de Peppel IP, Bodewes F, Moshage H, Fantin A, Farinati F, et al. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology. 2020; 72: 1864-1872.
- Ghoda A, Ghoda M. Liver Injury in COVID-19 Infection: A Systematic Review. Cureus. 2020; 12: e9487.
- Kumar MP, Mishra S, Jha DK, Shukla J, Choudhury A, Mohindra R, et al. Coronavirus disease (COVID-19) and the liver: a comprehensive systematic review and meta-analysis. Hepatol Int. 2020; 14: 711-722.
- Lenti MV, Borrelli de Andreis F, Pellegrino I, Klersy C, Merli S, Miceli E, et al. Impact of COVID-19 on liver function: results from an internal medicine unit in Northern Italy. Intern Emerg Med. 2020; 15: 1399-1407.
- Anastasiou OE, Korth J, Herbstreit F, Witzke O, Lange CM. Mild vs severe liver injury in SARS-CoV-2 infection. Dig Dis. 2020.
- Tian D, Ye Q. Hepatic complications of COVID-19 and its treatment. J Med Virol. 2020.
- Roedl K, Jarczak D, Drolz A, Wichmann D, Boenisch O, de Heer G, et al. Severe liver dysfunction complicating course of COVID-19 in the critically ill: multifactorial cause or direct viral effect? Ann Intensive Care. 2021; 11: 44.
- Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021; 41: 20-32.
- Liu J, Dong YQ, Yin J, He G, Wu X, Li J, et al. Critically ill patients with COVID-19 with ECMO and artificial liver plasma exchange: A retrospective study. Medicine (Baltimore). 2020; 99: e21012.
- Willars C. Update in intensive care medicine: acute liver failure. Initial management, supportive treatment and who to transplant. Curr Opin Crit Care. 2014; 20: 202-209.
- Weber S, Mayerle J, Irlbeck M, Gerbes AL. Severe liver failure during SARSCoV- 2 infection. Gut. 2020; 69: 1365-1367.
- Melquist S, Estepp K, Aleksandrovich Y, Lee A, Beiseker A, Hamedani FS, et al. COVID-19 presenting as fulminant hepatic failure: A case report. Medicine (Baltimore). 2020; 99: e22818.
- Gurala D, Al Moussawi H, Philipose J, Abergel JR. Acute Liver Failure in a COVID-19 Patient Without any Preexisting Liver Disease. Cureus. 2020; 12: e10045.
- Ali E, Ziglam H, Kohla S, Ahmed M, Yassin M. A Case of Fulminant Liver Failure in a 24-Year-Old Man with Coinfection with Hepatitis B Virus and SARS-CoV-2. Am J Case Rep. 2020; 21: e925932.
- Guo J, Xia H, Wang S, Yu L, Zhang H, Chen J, et al. The Artificial-Liver Blood-Purification System Can Effectively Improve Hypercytokinemia for COVID-19. Front Immunol. 2020; 11: 586073.
- Tandon R, Froghi S. Artificial liver support systems. J Gastroenterol Hepatol. 2020.
- Garcia Martinez JJ, Bendjelid K. Artificial liver support systems: what is new over the last decade? Ann Intensive Care. 2018; 8: 109.
- Kribben A, Gerken G, Haag S, Herget-Rosenthal S, Treichel U, Betz C, et al. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology. 2012; 142: 782- 789.e3.
