Abstract
Venous insufficiency is a severe complication of microvascular free tissue transfers, which may ultimately cause free flap failure. In this report, we describe a case of microvascular free flap reconstruction after oral cancer resection, complicated with venous thromboembolism manifested as venous insufficiency of the flap and pulmonary embolism (PE). Antithrombin III deficiency related heparin resistance was found during the course of treatment. With flap reexploration, veno-venous extracorporeal membrane oxygenation (V-V ECMO) and systemic anticoagulation therapy with a direct oral anticoagulant (DOAC), the flap was successfully salvaged and the patient recovered well.
Keywords: Venous thromboembolism; Free flap venous insufficiency; Pulmonary embolism; Microvascular free tissue transfer; Extracorporeal membrane oxygenation (ECMO); Direct oral anticoagulant (DOAC)
Case Presentation
A 59-year-old Asian man was admitted to the Division of the Oral and Maxillofacial Surgery of the Taipei Veterans General Hospital for a scheduled operation of right buccal squamous cell carcinoma and left retromolar trigon squamous cell carcinoma. He had class 1 obesity (Body Mass Index: 30.78kg/m²) and the history of a benign neoplasm of left buccal status post excision and reconstruction with a split-thickness skin graft seven years before this admission. He was a heavy smoker who had smoked half a pack of cigarettes every day for more than 20 years. He also chewed betel nuts and drank alcohol. No other medical history was noted. The pre-operative evaluation was acceptable. The American Society of Anaesthesiologists (ASA) physical status was level 2. Oral maxillofacial surgeons performed wide excision of the tumor, radical neck dissection, and tracheostomy for the patient, and then plastic surgeons harvested a free fibular flap from his left leg for reconstruction. The reconstruction was completed with one artery (the peroneal artery to the left superior thyroid artery) and one vein (a comitant vein to a branch of the left internal jugular vein) anastomosis. The whole operation had lasted for around 12 hours and was completed smoothly. The patient was then transferred to an intensive care unit for monitoring.
However, hypoxemia had been found since post-operative day (POD) 1. The chest film showed no pneumothorax, atelectasis, pulmonary infiltrate, or pleural effusion. There was only a small amount of sputum. We increased the setting of FiO2 and PEEP in order to keep oxygenation. On POD 3, flap swelling with a skin color change to purplish was noted (Figure 1). Under the impression of venous insufficiency of the flap, we re-explored the flap immediately at bedside and found engorgement in the recipient vein with venous thrombosis. We repositioned the vein and started anticoagulation therapy with enoxaparin (Clexane®) 2000 IU Q12H. The venous congestion of the flap was resolved; however, hypoxemia deteriorated so much that we had to increase the setting of FiO2 to 75% to maintain the SpO2. The D-dimer level was 1.153ug/ml at that time. Computed tomography pulmonary angiography (CTPA) was arranged for suspected pulmonary embolism (PE) on POD 3. The CTPA showed filling defects in the right main pulmonary artery to the inferior branch of the right pulmonary artery (Figure 2), and PE was diagnosed. A high dose of enoxaparin (Clexane®) 6000 IU Q12H was prescribed. Unfortunately, hypoxemia was still profound even under the anticoagulation therapy. Thus, we consulted a cardiovascular surgeon for veno-venous extracorporeal membrane oxygenation (V-V ECMO). Femoral-femoral V-V ECMO was set up on POD 5, and the continuous heparin infusion was used for thromboembolism prophylaxis. However, we found that the therapeutic level of heparin (aPTT > 55 seconds) could not be reached even under a high dose of heparin (30000 U/24 hours, 14U/kg/hr, aPTT: maximum to 32.8 seconds). In the survey of heparin resistance, antithrombin III deficiency was found (antithrombin III: 55.2%, reference range: 83~128%). Due to heparin resistance, we shifted the heparin pump to Rivaroxaban 15mg BID on POD 9. Under the treatment of a direct oral anticoagulant (DOAC), his respiratory condition showed gradual improvement. The patient successfully weaned from VV-ECMO on POD 13 and a ventilator on POD 14.
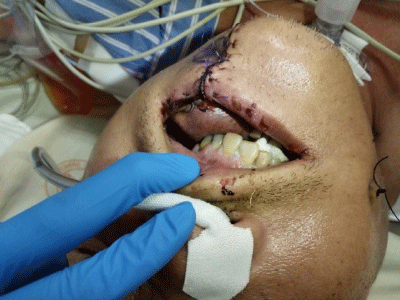
Figure 1: An edematous change and a purplish color of the flap were noted
on POD 3. Venous insufficiency of the flap was impressed.
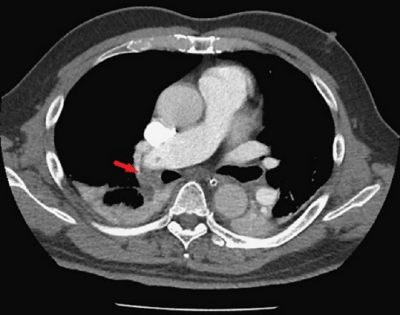
Figure 2: The arrow labeled the filling defects in the right main pulmonary
artery to the inferior branch of the right pulmonary artery. Pulmonary
embolism was considered.
The following hospital course was smooth, and the condition of the flap was also good. The patient was discharged in a stable condition.
Discussion
Venous thromboembolism (VTE) includes a spectrum of diseases, ranging from asymptomatic deep vein thrombosis (DVT) to fatal pulmonary embolism (PE). VTE is a complex and multifactorial disease, influenced by acquired or inherited predispositions to thrombosis and VTE risk factors [1]. Heit et al. reported several independent risk factors for VTE in a US population-based study, including surgery, malignant neoplasms with or without chemotherapy, trauma, hospital or nursing home confinement, central venous catheters or pacemakers, superficial vein thrombosis, and neurological disease with extremity paresis [2]. In addition, increasing patient age, obesity (BMI ≥35kg/m²), an increased baseline plasma fibrin D-dimer, and a family history of VTE had been identified as independent risk factors for VTE [3,4].
VTE can be a severe complication related to cancer surgery and may cause significant postoperative morbidity and mortality. Early detection and treatment of VTE is important.
In the last few years, there have been many advances in the field of PE. Some management, including local thrombolysis, mechanical extraction devices, hemodynamic support devices, such as extracorporeal membrane oxygenation and surgical embolectomy, has been introduced [5]. According to the consensus practice from the Pulmonary Embolism Response Team (PERT) Consortium, highrisk PE is defined hemodynamically as acute PE with hypotension (systolic blood pressure [SBP] <90mmHg for >15 minutes, a drop in SBP of 40mmHg or more from the baseline, or requiring hemodynamic support). Mechanical circulatory support, e.g., venoarterial extracorporeal membrane oxygenation (V-A ECMO), should be considered for patients with high-risk PE and cardiogenic shock with cardiac arrests or refractory shock to low-dose inotropes or with failure of thrombolysis. Mechanical circulatory support can also be used in combination with multiple treatment modalities, such as systemic thrombolysis, catheter-directed therapy, or surgical pulmonary embolectomy [6].
In case of massive PE, the primary approach is usually V-A ECMO; however, V-V ECMO can be used in the selected cases with pulmonary failure aggravating right ventricular strain [7]. In the retrospective study conducted by Federico Sertic et al., 36 patients required ECMO placement (32 V-A and 4 V-V ECMO) following acute PE and the overall survival to hospital discharge was 44.4% (16/36). Survival to hospital discharge was 50% (2/4) in the V-V ECMO group, and both patients were alive in the two-year follow-up [8]. In another series from Paul Maggio et al., 21 patients received extracorporeal life support (ECLS) for massive pulmonary emboli from January 1992 to December 2005, and V-V ECMO was used in two patients (patient number 7 and 13) because they had adequate perfusion on vasoactive drugs and mild right ventricular strain on echocardiograms. One was a 26-year-old male who suffered from cardiopulmonary deterioration a week after a motor vehicle crash, and the other one was a 53-year-old female who had antithrombin III deficiency, and post-operative PE was noted after an ankle surgery. Both patients survived and were discharged [9]. In addition to these two studies, some case reports had confirmed the utilization of V-V ECMO in PE with consecutive pulmonary failure [10-14]. In our case, a timely activated ECMO team helped us stabilize the patient, which enabled us to initiate the subsequent systemic anticoagulation therapy for him.
Early therapeutic anticoagulation, initiated even prior to the confirmed diagnosis when there is high clinical suspicion of acute PE, has been proved to improve mortality and decrease recurrence. When additional therapeutic modalities are considered, anticoagulation should not be delayed unless contraindicated [6].
In the retrospective study investigating internal jugular vein thrombosis (IJVT) and PE after head and neck reconstructive surgery conducted by D. Kitano et al., 23 patients were diagnosed with IJVT, and PE occurred among two of them. One patient had clinical symptoms, including chest pain and dyspnea, but the other one was asymptomatic. Both patients received three months of anticoagulation therapy with DOAC, and there were no hemorrhagic events or recurrence of PE during the anticoagulation therapy. In addition, the follow-up CT scans revealed the disappearance of thrombus [15,16].
Conclusion
Venous thromboembolism, including a spectrum of diseases ranging from asymptomatic deep vein thrombosis to life-threatening pulmonary embolism, is a complex and multifactorial disease. In the field of microvascular free tissue transfers, venous thromboembolism, or venous insufficiency, is devasting to a free flap and may lead to inadequate arterial inflow, flap ischemia, and eventual necrosis. Early detection and timely treatment, in combination with different methods according to the clinical scenarios, are of the utmost importance.
References
- Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016; 41: 3-14.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a populationbased case-control study. Arch Intern Med. 2000; 160: 809-815.
- Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015; 12: 464-474.
- Barsoum MK, Heit JA, Ashrani AA, Leibson CL, Petterson TM, Bailey KR. Is progestin an independent risk factor for incident venous thromboembolism? A population-based case-control study. Thromb Res. 2010; 126: 373-378.
- Essien EO, Rali P, Mathai SC. Pulmonary Embolism. Med Clin North Am. 2019; 103: 549-564.
- Rivera-Lebron B, McDaniel M, Ahrar K, Alrifai A, Dudzinski DM, Fanola C, et al; PERT Consortium. Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium. Clin Appl Thromb Hemost. 2019; 25: 1076029619853037.
- Kmiec L, Philipp A, Floerchinger B, Lubnow M, Unterbuchner C, Creutzenberg M, et al. Extracorporeal Membrane Oxygenation for Massive Pulmonary Embolism as Bridge to Therapy. ASAIO J. 2020; 66: 146-152.
- Sertic F, Diagne D, Chavez L, Richards T, Berg A, Acker M, et al. Midterm outcomes with the use of extracorporeal membrane oxygenation for cardiopulmonary failure secondary to massive pulmonary embolism. Eur J Cardiothorac Surg. 2020; 58: 923-931.
- Maggio P, Hemmila M, Haft J, Bartlett R. Extracorporeal life support for massive pulmonary embolism. J Trauma. 2007; 62: 570-576.
- Giraud R, Banfi C, Siegenthaler N, Bendjelid K. Massive pulmonary embolism leading to cardiac arrest: one pathology, two different ECMO modes to assist patients. J Clin Monit Comput. 2016; 30: 933-937.
- Seaton A, Hodgson LE, Creagh-Brown B, Pakavakis A, Wyncoll D, Doyle JF. The use of veno-venous extracorporeal membrane oxygenation following thrombolysis for massive pulmonary embolism. J Intensive Care Soc. 2017; 18: 342-347.
- Faggian G, Onorati F, Chiominto B, Gottin L, Dan M, Ribichini F, et al. Venovenous extracorporeal membrane oxygenation as a bridge to and support for pulmonary thromboendarterectomy in misdiagnosed chronic thromboembolic pulmonary hypertension. Artif Organs. 2011; 35: 956-960.
- Fernandes P, Allen P, Valdis M, Guo L. Successful use of extracorporeal membrane oxygenation for pulmonary embolism, prolonged cardiac arrest, post-partum: a cannulation dilemma. Perfusion. 2015; 30: 106-110.
- Aitchison S, Hoopes CW, Roth JS. Venovenous extracorporeal membrane oxygenation for the treatment of acute pulmonary embolism after ventral hernia repair. Am Surg. 2013; 79: 444-446.
- Kitano D, Yonezawa K, Iwae S, Sakakibara S. Internal jugular vein thrombosis and pulmonary thromboembolism after head and neck reconstructive surgery. J Plast Reconstr Aesthet Surg. 2021; 74: 1239-1245.
- Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: A review. Open J Prev Med. 2012; 2: 499-509.
Citation:Shih-Hao M and Szu-Hsien W. Successful Salvage of Venous Thromboembolism Manifested as Free Flap Venous Insufficiency and Pulmonary Embolism in a Patient Receiving Microvascular Free Flap Reconstruction after Oral Cancer Resection: A Case Report and Review of the Literature. Austin Crit Care Case Rep. 2022; 6(1): 1038.
