Abstract
We report a case of a patient suffering from severe COVID-19 complicated by CAPA (COVID-19 associated pulmonary aspergillosis). Successful therapy with systemic voriconazole and isavuconazole failed, liposomal Amphotericin B was not possible due to acute renal failure. After nebulized Amphotericin was added to systemic isavuconazole, CAPA could be treated successfully.
Keywords: COVID-19; CAPA; Invasive Aspergillosis; Amphotericin B
Case Report
A 52-year-old woman, whose past medical history included obesity, arterial hypertension, diabetes mellitus II, peripheral arterial occlusive disease necessitating transtibial amputation, was admitted to the normal ward of our department of infectious diseases in July 2021 due to dyspnoea and fatigue for the last four days. An X-ray showed bilateral pneumonic infiltrations, the RT-PCR from a nasopharyngeal swab was SARS-CoV-2 positive (L452R und T478K, CT value 26. 1). The initial laboratory results showed normal white blood cell count (WBC) of 6 G/l with a 18% lymphocytes, an elevated C-reactive protein (CRP) of 131 mg/l and IL 6 of 284 pg/mL). Due to respiratory failure oxygen was administered via nasal cannula, however, one day later, the patient had to be transferred to the intensive care unit. Noninvasive ventilation was started, and an immunomodulating therapy with dexamethasone 20mg for 5 days, followed by 10mg for 5 days was given. Three days after hospital admission intubation and mechanical ventilation were necessary. A total five cycles of prone positioning were performed. Seven days after hospital admission inflammation parameterswere rising (WBC 16 G/L, CRP 344. 4 mg/l, IL 6 322 pg/ mL) and fever spiked up to 40°C. The X-ray showed a deterioration of the infiltrates. Piperacillin/Tazobactam was initiated empirically. Multiplex PCR of tracheal secretion detected no pathogen, and there was no growth in the blood cultures. A bronchoscopy showed white/greyish plaques in both main bronchi (see picture 1a). Galactomannan (GM) from bronchoalveolar lavage (BAL) was highly positive (ODI over the measurable value), and cultures grew Aspergillus fumigatus complex. Also, Beta-D-Glucan (BDG) from serum was highly positive (> 500pg/ml). A therapy with voriconazole (VOR) 6mg/kg bid on day 1, followed by 4mg/kg bid on day 2 was started. After three days therapy was switched to isavuconazole (ISA) (200 mg tid for two days, followed by 200 once daily) due acute renal failure and rising of liver function tests. Oral administration was not possible due massive regurgitation over the gastric tube. Initially,the inflammation parameters declined slightly. However, four days later the respiratory situation deteriorated, inflammation parameters increasedsharply (WBC 17 G/L, CRP 402. 7 mg/l, IL 6 340 pg/mL). A SARS-CoV-2 PCR of tracheal secretion repeatedly showed a CT value over 30, so a successful clearance of SARSCoV- 2 was assumed. Bronchoscopy 10 days after start of antifungal therapy macroscopically showed an impressive deterioration: Tracheobronchial ulcerations with pseudomembranes and white plaques were observed (see picture 1b). GM was again highly positive (ODI over the measurable value), aspergillus fumigatus complex grew in all cultures of BAL. A CT showed bilateral infiltrates (see picture 2), but there was no sign of caverns, organised pneumonia or pulmonary embolism. As the respiratory situation deteriorated further, prone positioning was started again. A tracheotomy was performed. The antimicrobial resistance testing showed susceptibility to ISA, VOR and Amphotericin B. Nebulizedliposomal Amphotericin B (lipAMB) (Ambisome®, Gilead) was added to intravenous ISA (inhalation over 30 minutes via a nebulizer of 25mg lipAMP attenuated with aqua). The nebulized therapy was tolerated well. GM of BAL decreased continuously (ODI 5. 4 after 6 days, ODI 3. 6 after 12 days and negative after 30 days of inhalative therapy). A bronchoscopy 30 days after initiation of inhaled therapy showed great improvement (Figure 1c). Weaning and mobilisation could be done successfully and decannulation was performed 55 days after hospital admission. The inflammation parameters decreased to normal values, the kidney parameters normalised. Inhaled lipAMB and intravenous ISA were stopped and oral VOR started (2x400mg once followed by 2x200mg for 8 weeks). The VORserum level was always within the range of 2-4 mcg/mL. BDG from serum decreased continuously (320 pg/ml after 38 days and 170 pg/ml after 45 days of antifungal therapy).
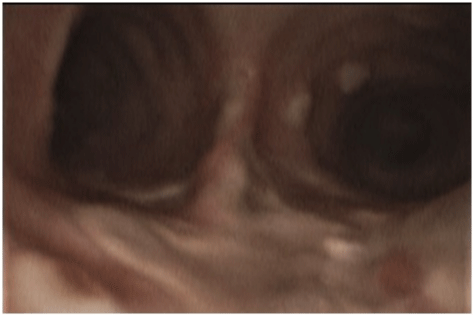
Figure 1a: Bronchoscopic images before starting antifungal therapy.
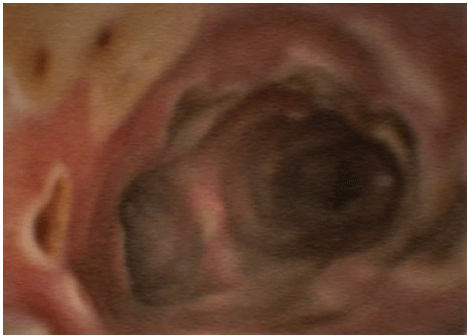
Figure 1b: After 10 days of IV antifungal therapy.
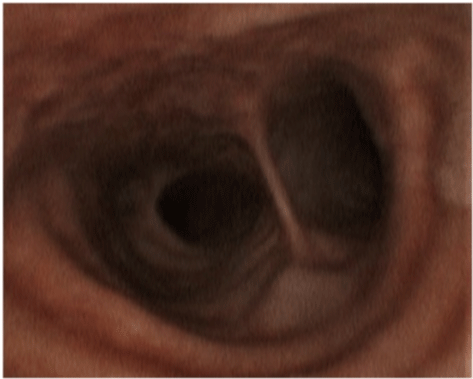
Figure 1c: After 30 days of nebulized and IV antifungal therapy.
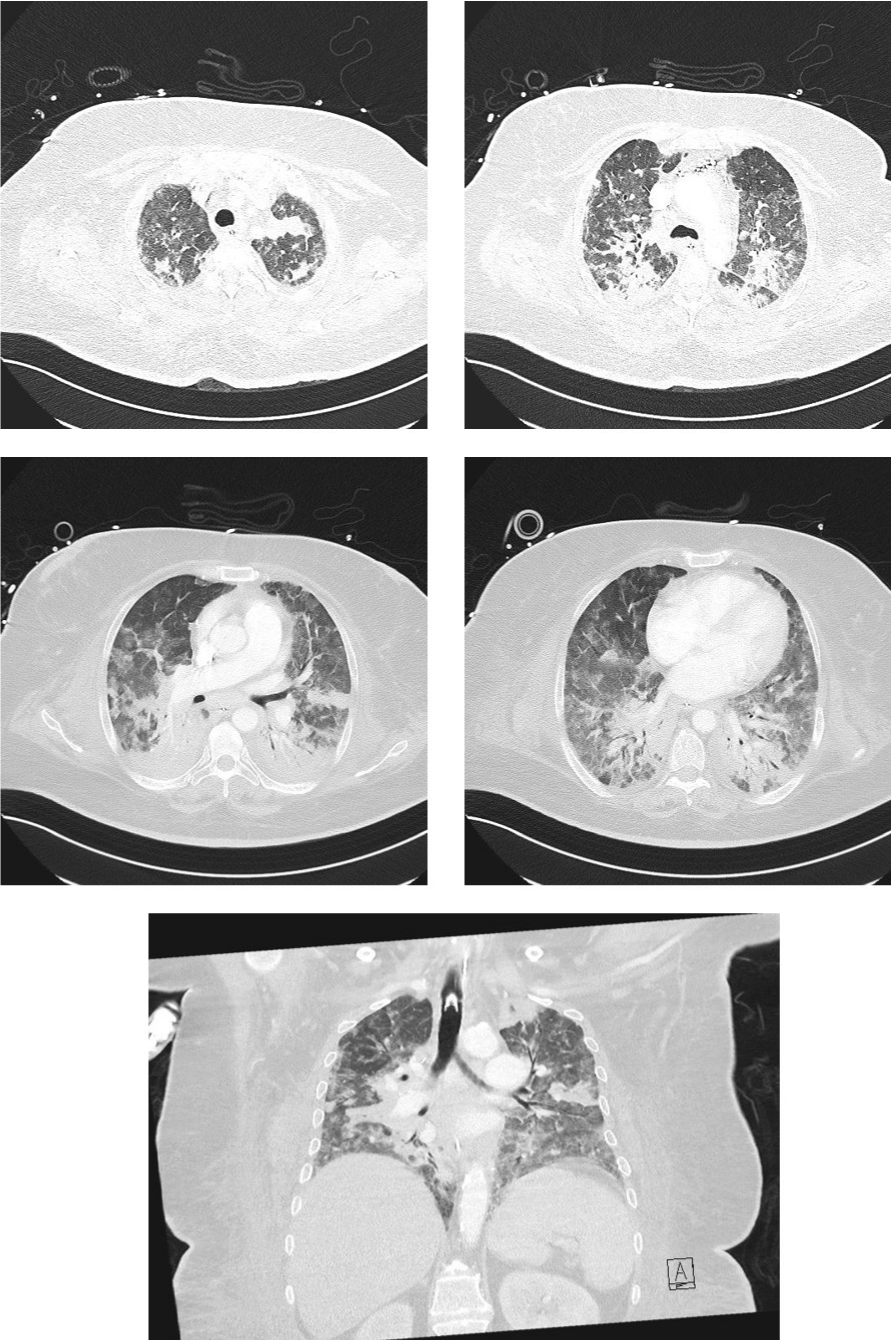
Figure 2: The computertomography shows for COVID-19 typical alterations,
like bilateral consolidations with ground-glass opacities and vascular
enlargement.
The patient was transferred to the normal ward after 56 days in the intensive care unit and 60 days after symptom onset and was discharged to a rehabilitation centre 6 days later without signs of relapse of aspergillosis. Invasive aspergillosis was treated for a total 102 days with VOR or ISA and 33 days of nebulized lipAMB.
Review
COVID-19 associated invasive aspergillosis (CAPA) is a severe complication of COVID-19 with a reported incidence between 26-35% [1,2] in patients with ARDS, however qualitative data is scarce. Due to the high mortality [3], adequate therapy is crucial. The recommended first line therapy for invasive aspergillosis is voriconazole. However, administration of intravenous VOR is related with several side effects. In our case, liver function tests and kidney parameters worsened. Further, wide variations of VOR plasma levels are seen in critical ill patients making frequent changes in drug doses necessary [3]. Isavuconazol has demonstrated to be non-inferior to VOR and side effects are less common, so we switched to intravenous ISA [4].
Unlike VOR, routine therapeutic drug monitoring is not recommended for ISA. However, there are some studies suggesting that it might be necessary in some cases. For example, female gender and obesity were identified as risk factors for low plasma levels [5,6]. An explanation for the gender related difference is that over 60% of ISA is metabolized by hepatic CYP3A [7,8], and women have greater hepatic CYP3A activity than men [9]. Another factor might be that the protein binding of ISA is over 99%. In patients having hypoalbuminemia, clearance of ISA is likely increased due to an increased unbound fraction of ISA [5].
We are suggesting that in our case, the plasma level of ISA was too low given that the patient was female, obese and had severe hypoalbuminemia. Interestingly, we did not find any studies evaluating ISA plasma levels in critical ill patients. Factors like capillary leak and interaction with other drugs might have an impact in ISA levels as well. Unfortunately, there was no possibility to measure ISA levels in our department.
In our patient, adding intravenous LipAMB to ISA was not an option, given that the patient suffered from acute renal failure at this point of time and systemic lipAMP has nephrotoxic potential. Studies with mice and rats demonstrated that nebulized AMB is efficient regarding treatment and prevention of invasive aspergillosis [10–18]. High lung tissue concentrations and low systemic exposure were seen [10,13,16,17,19]. Inhalation of LipAMB showed longer lung retention than standard AMB [11].
There is a lack of human studies using nebulized lipAMB. Some studies reported a decrease of incidence of invasive aspergillosis if used prophylactically in hematological patients with chemotherapyinduced prolonged neutropenia [20,21]. There are some case reports demonstrating successful treatment of invasive aspergillosis with inhaled lipAMB and systemic antifungal therapy [22]. However, due the concomitant administration of other antifungal therapy, the impact of nebulizedlipAMB is unclear. In all studies the inhalation of lipAMB was described as well tolerated, except for some mild effects related topulmonary irritation [22].
In our case nebulized AMB was well tolerated and together with an azole led to a successful therapy of severe CAPA. We suggest considering this therapeutic option in patients with lack of clinical response or if systemic therapy is not possible due to side effects. Furthermore, we believe that future studies are crucial to evaluate the impact of therapeutic drug monitoring of ISA in critically ill patients.
Declarations
Conflict of interest: There are no conflicts of interest.
Funding: There was no funding.
Patient’s consent: Patient gave written consent for the publication of the manuscript and pictures.
Acknowledgments: Not applicable.
Contribution: All authors contributed significantly to the manuscript.
References
- Koehler P, Cornely OA, Böttiger BW, Dusse F, Eichenauer DA, Fuchs F, et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020; 63(6): 528-534. doi:10.1111/myc.13096.
- Rutsaert L, Steinfort N, Hunsel TV, Bomans P, Naesens R, Mertes H, et al. COVID-19-associated invasive pulmonary aspergillosis. Annals of Intensive Care. 2020; 10(1). doi:10.1186/s13613-020-00686-4.
- Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. The Lancet. Infectious Diseases. 2020; 21(6): e149-e162. doi:10.1016/ S1473-3099(20)30847-1.
- Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. The Lancet. 2016; 387(10020): 760-769. doi:10.1016/S0140-6736(15)01159-9.
- Wu X, Clancy CJ, Rivosecchi RM, Zhao W, Shields RK, Marini RV, et al. Pharmacokinetics of Intravenous Isavuconazole in Solid-Organ Transplant Recipients. Antimicrobial Agents and Chemotherapy. 2018; 62(12). doi:10.1128/AAC.01643-18.
- Andes D, Kovanda L, Desai A, Kitt T, Zhao M, Walsh TJ. Isavuconazole Concentration in Real-World Practice: Consistency with Results from Clinical Trials. Antimicrobial Agents and Chemotherapy. 2018; 62(7). doi:10.1128/ AAC.00585-18.
- Townsend R, Dietz A, Hale C, Akhtar S, Kowalski D, Lademacher C, et al. Pharmacokinetic Evaluation of CYP3A4-Mediated Drug-Drug Interactions of Isavuconazole With Rifampin, Ketoconazole, Midazolam, and Ethinyl Estradiol/Norethindrone in Healthy Adults. Clinical Pharmacology in Drug Development. 2017; 6(1): 44-53. doi:10.1002/cpdd.285.
- Groll AH, Desai A, Han D, Howieson C, Kato K, Akhtar S, et al. Pharmacokinetic Assessment of Drug-Drug Interactions of Isavuconazole With the Immunosuppressants Cyclosporine, Mycophenolic Acid, Prednisolone, Sirolimus, and Tacrolimus in Healthy Adults. Clinical Pharmacology in Drug Development. 2017; 6(1): 76-85. doi:10.1002/cpdd.284.
- Hu Z, Zhao Y. Sex-Dependent Differences in Cytochrome P450 3A Activity as Assessed by Midazolam Disposition in Humans: A Meta-Analysis. Drug Metabolism and Disposition. 2010; 38(5): 817-823. doi:10.1124/ dmd.109.031328.
- Sorensen KN, Allent SD, Nejd MJ, Proffitt RT. Aerosolization of liposomal (AmBisome®) and non-liposomal (Fungizone®) amphotericin B as A treatment for pulmonary fungal infections. Journal of Controlled Release. 1994; 28(1–3).
- Allen SD, Sorensen KN, Nejdl MJ, Durrant C, Proffit RT. Prophylactic efficacy of aerosolized liposomal (AmBisome) and non-liposomal (Fungizone) amphotericin B in murine pulmonary aspergillosis. The Journal of antimicrobial chemotherapy. 1994; 34(6): 1001-1013. doi:10.1093/JAC/34.6.1001.
- Ruijgrok EJ, Vulto AG, Etten EWV. Efficacy of aerosolized amphotericin B desoxycholate and liposomal amphotericin B in the treatment of invasive pulmonary aspergillosis in severely immunocompromised rats. The Journal of antimicrobial chemotherapy. 2001; 48(1): 89-95. doi:10.1093/JAC/48.1.89.
- Schmitt HJ, Bernard EM, Häuser M, Armstrong D. Aerosol amphotericin B is effective for prophylaxis and therapy in a rat model of pulmonary aspergillosis. Antimicrobial Agents and Chemotherapy. 1988; 32(11): 1676- 1679. doi:10.1128/AAC.32.11.1676.
- Gavalda` J, Martin M, Lopez P, Gomis X, Ramirez J, Rodriguez D, et al. Efficacy of Nebulized Liposomal Amphotericin B in Treatment of Experimental Pulmonary Aspergillosis. Antimicrobial Agents and Chemotherapy. 2005; 49(7): 3028-3030. doi:10.1128/AAC.49.7.3028-3030.2005.
- Gilbert BE. Liposomal aerosols in the management of pulmonary infections. Journal of Aerosol Medicine. 1996; 9(1): 111-22.
- Ruijgrok EJ, Fens MHA, Bakker-Woudenberg IAJM, Etten EWM, Ruijgrok EJ, Vulto AG. Nebulization of four commercially available amphotericin B formulations in persistently granulocytopenic rats with invasive pulmonary aspergillosis: evidence for long-term biological activity. Journal of Pharmacy and Pharmacology. 2005; 57(10): 1289-1295. doi:10.1211/jpp.57.10.0007.
- Olson JA, Adler-Moore JP, Schwartz J, Jensen GM, Proffitt RT. Comparative Efficacies, Toxicities, and Tissue Concentrations of Amphotericin B Lipid Formulations in a Murine Pulmonary Aspergillosis Model. Antimicrobial Agents and Chemotherapy. 2006; 50(6): 2122-2131. doi:10.1128/AAC.00315-06.
- Ho KM, Duff O, Chambers D, Murray R. Meta-analysis of nebulized amphotericin B to prevent or treat pulmonary aspergillosis in immunosuppressed animals. Transplant Infectious Disease. 2008; 10(3): 168-76.
- RUIJGROK EJ, VULTO AG, ETTEN EWM. Aerosol Delivery of Amphotericin B Desoxycholate (Fungizone) and Liposomal Amphotericin B (AmBisome): Aerosol Characteristics and In-vivo Amphotericin B Deposition in Rats. Journal of Pharmacy and Pharmacology. 2000; 52(6): 619-627. doi:10.1211/0022357001774417.
- Peghin M, Monforte V, Martin-Gomez M, Ruiz-Camps I, Berastegui C, Saez B, et al. 10 years of prophylaxis with nebulized liposomal amphotericin B and the changing epidemiology of Aspergillus spp. infection in lung transplantation. Transplant International. 2016;29(1):51-62. doi:10.1111/tri.12679
- Rijnders BJ, Cornelissen JJ, Slobbe L, Becker MJ, Doorduijn JK, Hop WCJ, et al. Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: a randomized, placebo-controlled trial. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008; 46(9): 1401-1408. doi:10.1086/586739.
- Kuiper L, Ruijgrok EJ. A review on the clinical use of inhaled amphotericin B. Journal of aerosol medicine and pulmonary drug delivery. 2009; 22(3): 213- 227. doi:10.1089/jamp.2008.0715.
