
Case Report
Austin J Emergency & Crit Care Med. 2014;1(2): 4.
T-cell Acute Lymphoblastic Leukemia Presenting as a Cardiac Tamponade
El Sayed MJ1*, Gharzuddin W2, Kharfan-Dabaja MA3
1Department of Emergency Medicine, American University of Beirut Medical Center, Lebanon
2Department of Internal Medicine, Division of Cardiology, American University of Beirut Medical Center, Lebanon
3Department of Blood and Marrow Transplantation, Moffitt Cancer Center, USA
*Corresponding author: El Sayed MJ, Department of Emergency Medicine, American University of Beirut Medical Center, PO. Box: 11-0236 Riad El Solh, Beirut 1107 2020, Lebanon
Received: December 02, 2014; Accepted: December 18, 2014; Published: December 22, 2014
Abstract
Acute leukemia presenting as a clinically significant pericardial effusion or cardiac tamponade is a rare event. We report the case of a young man who presented to the emergency department with symptoms of dyspnea and hemoptysis and was found to have a cardiac tamponade as the presenting sign of a hematologic malignancy. The patient underwent a therapeutic pericardiocentesis which showed presence of lymphoblasts. A bone marrow aspiration and phenotype analysis confirmed the diagnosis of T-cell acute lymphoblastic leukemia. He was started on chemotherapy without immediate complications. Additionally, we briefly describe the approach to diagnosing pericardial effusions in complex emergency presentations by raising clinical suspicion and identifying suggestive signs on the different tests available to the emergency physician.
Keywords: T-cell; Leukemia; Pericardial effusion; Tamponade; Dyspnea
Abbreviations
ALL: Acute Lymphoblastic Leukemia; CD: Cluster of Differentiation; CT: Computer Tomography; ECG: Electrocardiogram; ED: Emergency Department; HIV: Human Immunodeficiency Virus; LDH: Lactate Dehydrogenase; RT-PCR: Real Time Polymerase Chain Reaction; TdT: Terminal deoxynucleotidyl Transferase; TEL-AML1 (T 12,21): Fusion gene resulting from chromosomal translocation t (12,21); E2A-PBX (T 1,19): Fusion gene resulting from chromosomal translocation t (1,19); BCR-ABL (T 9,22): Fusion gene resulting from chromosomal translocation t (9,22); AF4-MLL (T 4,11): Fusion gene resulting from the chromosomal translocation t (4;11)
Case Presentation
A 27 year old male, previously healthy, presented to the emergency department (ED) with progressive dyspnea and productive cough of one week duration. His symptoms started one month prior to presentation, mainly manifesting as a non-productive cough and nasal congestion for which he received several courses of antibiotics on an outpatient basis for a presumed diagnosis of bronchitis. On further review of systems the patient reported low-grade fever, night sweats, two episodes of hemoptysis, and unintentional weight loss of 5 kg over one month duration. He denied any chest pain or skin rashes. His social history was relevant for recent travel to Africa for work without exposure to livestock or livestock products.
On presentation the patient's vital signs showed tachycardia (heart rate of 135/min) and tachypnea (respiratory rate of 22/min). He was afebrile (37°C), his blood pressure was 168/106 mmHg and his oxygen saturation was 99% on room air. His physical examination was notable for decreased breath sounds in left lower lung base and for tachycardia with a systolic ejection murmur on auscultation. The rest of the physical examination was unremarkable for palpable lymphadenopathy or organomegaly.
In the ED, the patient was placed on droplet precautions isolation, pertinent laboratory tests were ordered, and intravenous (IV) fluid resuscitation was started. Electrocardiogram (ECG) demonstrated sinus tachycardia with electrical alternans (Figure 1). Chest radiograph showed cardiomegaly and a left lower lobe lung consolidation (Figure 2). A bedside cardiac ultrasound showed pericardial effusion with mild diastolic right ventricular collapse and evidence of respiratory variation of mitral inflow velocities consistent with tamponade physiology. The ultrasound also revealed the presence of a left hilar mass. A chest CT with IV contrast confirmed the presence of a large mediastinal heterogeneous mass (11.8 x 4.4 x 4.8 cm) (Figure 3) compressing the pulmonary artery and the left main bronchus, in addition to a large pericardial effusion (Figure 4) and splenomegaly. The initial laboratory tests were notable for anemia (hemoglobin 8.6 g/dL), thrombocytopenia (35,000 /μL) and a blood film showing presence of blasts (Table 1). His coagulation profile and biochemical profile were within desirable range except for an elevated lactate dehydrogenase test (LDH 2093 IU/L).
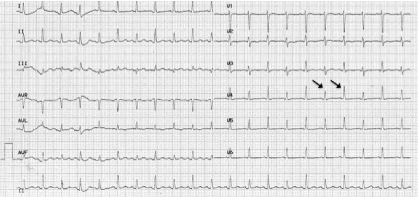
Figure 1: ECG.
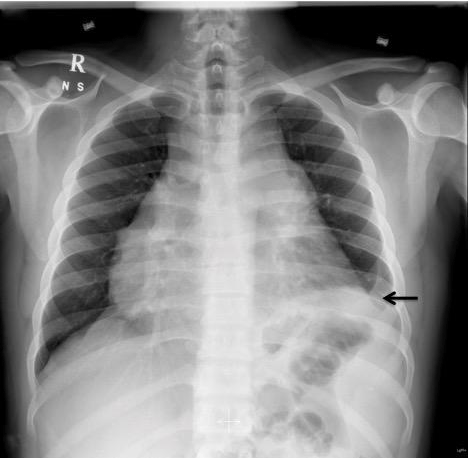
Figure 2: CXR showing cardiomegaly and left lower lobe consolidation.
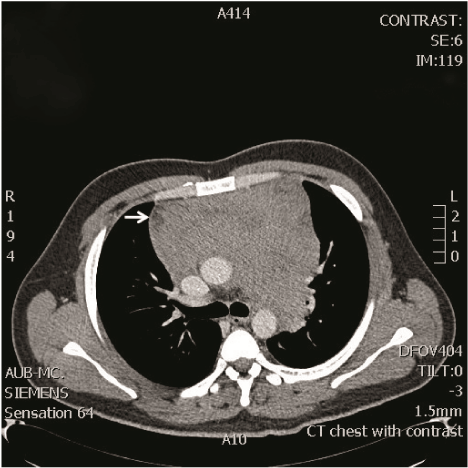
Figure 3: Chest CT showing a large heterogeneous mass involving the superior and anterior mediastinum, measuring approximately 11.8 x 4.4 x 4.8 cm.
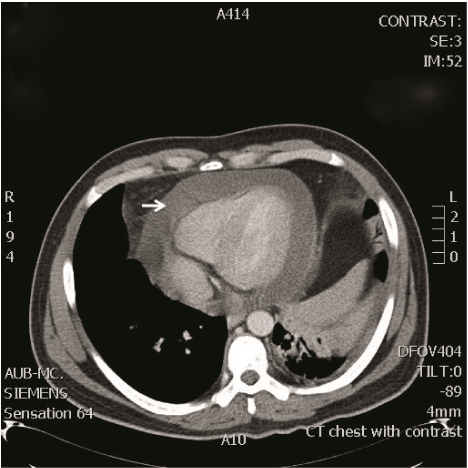
Figure 4: Chest CT showing large pericardial effusion.
a. CBC*, AUTOMATED
WBCs
10100
/cu.mm
[4000 - 11000]
RBCs
2.91
mil/mm3
[Female 4.00-5.50]
[Male 4.50-6.50]HEMOGLOBIN
8.6
g/dL
[Female 12.0-16.0]
[Male 13.0-18.0]HEMATOCRIT
24.0
%
[Female 37.0-46.0]
[Male 40.0-54.0]MCV
83.0
fl
[80.0 - 94.0]
MCH
30.0
pg
[27.0 - 31.0]
MCHC
36.0
g/dL
[30.0 - 35.0]
RDW
14.0
%
[11.6 - 14.6]
WBCs DIFFERENTIAL COUNT
POLYMORPHONUCLEARS
29
%
[40 - 65]
BANDS
2
%
[0 - 2]
LYMPHOCYTES
32
%
[25 - 40]
MONOCYTES
14
%
[2 - 8]
EOSINOPHILS
2
%
[0 - 4]
BASOPHILS
2
%
[0 - 1]
BLASTS
4
%
[0]
PROMYELOCYTES
5
%
[0]
MYELOCYTES
6
%
[0]
METAMYELOCYTES
4
%
[0]
NUCL. RBCs
19
/100WBC
PLATELET COUNT
35000
/cu.mm
[150000 - 400000]
ABSOLUTE NEUTROPHIL COUNT
3131
/cu.mm
[1600 - 7200]
b. BLOOD FILM INSPECTION
RBCs: Slight anisocytosis and polychromasia. Many NRBCs seen.
WBCs: Left shifted granulocytes, monocytosis and some blasts seen.
Platelets: Thrombocytopenia.
Table 1: Laboratory Tests. * Complete Blood Count.
The patient was evaluated in the ED by the cardiology team for early pericardiocentesis and by the hematology oncology team for the possibility of a lymphoproliferative disorder. He was admitted to the coronary care unit; and, after receiving platelets transfusion he underwent a pericardiocentesis with aspiration of 600 cc of serosanguineous fluid (Table 2) followed by placement of a pigtail catheter. The patient also underwent a bone marrow aspiration. Morphologic as well as flow-cytometry analysis of the pericardial fluid and marrow aspirate showed presence of 4% and 77% T-cell lymphoblasts, respectively, showing positivity for CD10, dim CD1a, CD3, CD4, CD5, CD7, CD47 and TdT while negativity for CD2, CD8 and CD34. Conventional chromosome banding showed normal male karyotype (46, XY). RT-PCR were negative for TEL-AML1 (T 12,21), E2A-PBX (T 1,19), BCR-ABL (T 9,22), and AF4-MLL (T 4,11). Further workup also included negative cultures, negative HIV serology, tuberculin skin test and malaria smear test. During his hospital stay, the patient received a course of antibiotics (cefepime and levofloxacin) for community acquired pneumonia and was started on the augmented Berlin-Frankfurt-Munster (BFM) regimen [1] without immediate complications. A repeat echocardiograph showed resolution of the pericardial effusion and the catheter was removed. Six weeks later, he presented with high grade fever associated with dyspnea and palpitations. Work up showed a moderate pericardial effusion, raising concerns for the possibility of a pyogenic pericarditis following a diagnostic pericardiocentesis. Consequently, he underwent a therapeutic pericardiectomy without post surgical complications. At the time of this report, the patient is completing his prescribed post-induction treatment without evidence of persistent T-ALL.
Chemistry Profile
a. Pericardial Fluid:
GLUCOSE (BODY FLUID)
74
mg/dL
PROTEIN (BODY FLUID)
58
g/L
ALBUMIN (BODY FLUID)
37
g/L
LDH (BODY FLUID)
2457
IU/L
b. Serum
PROTEIN A/G
60
g/L
[60 - 83]
ALBUMIN
34
g/L
[36 - 53]
GLOBULIN
26
g/L
[24 - 30]
LDH
2093
IU/L
[110 - 265]
Table 2: Laboratory Tests: Biochemistry Profile.
Discussion
Clinically significant pericardial effusions and cardiac tamponade are life-threatening conditions that rarely constitute the presenting signs of leukemias. Several case reports exist in the literature describing new diagnoses of hematologic malignancies made after acute cardiac presentations [2-4]. More commonly, patients with known lymphomas or leukemias develop neoplastic effusions during the course of their chemotherapy treatment. A prevalence of pericardial effusion of up to 17% was previously described in lymphoma/ leukemia patients [5]. Higher prevalence was also reported with other types of malignancies (lung and breast) [5].
In additions to malignancies, other causes of new onset non-traumatic pericardial effusions include but are not limited to infectious causes (tuberculosis, HIV, viral pericarditis), hypothyroidism, autoimmune and rheumatologic diseases (Systemic lupus erythematous, rheumatoid arthritis, scleroderma, etc.), uremia, myocardial infarction, aortic dissection, postoperative or post-procedural (cardiac surgery) and idiopathic causes [6-10]. A thorough history and physical examination can help rule out some of these etiologies in the ED. Our patient's presentation was initially concerning for pneumonia and more specifically for tuberculosis (dyspnea, hemoptysis and recent residence in a tuberculosis endemic area) or for pulmonary emboli. The ED workup however helped narrow down the diagnosis to a hematologic malignancy.
Dyspnea is the most common complaint in patients who are symptomatic from a pericardial effusion. Chest pain and fullness among other nonspecific complaints such as fever and lethargy may also be present [9]. Physical examination findings of clinically significant pericardial effusion may include jugular venous distention, tachycardia, deep heart sounds and pulsusparadoxus [11]. The presence of Beck's triad (muffled heart sounds, hypotension and jugular venous distention) should raise the suspicion for cardiac tamponade. This classic presentation is not however always present in cardiac tamponade [12].
ECG findings suggesting the presence of a large effusion include low voltage, electrical alternans and PR segment depression [13]. Cardiomegaly on chest radiograph has also been found to be fairly sensitive for the presence of a large effusion or cardiac tamponade [11].
Doppler echocardiography remains the gold standard tool for diagnosing pericardial effusions and cardiac tamponade. Emergency physicians with formal ultrasound training can perform bedside focused cardiac ultrasound relatively quickly with good sensitivity, specificity and accuracy for detecting pericardial effusions [14-15]. Upon detection of a pericardial effusion on echocardiography, the emergency physician should look for diagnostic signs of tamponade physiology such as chamber collapses of the right atrium and right ventricle during diastole [16]. One should also keep an eye open for the possibility of finding on ultrasound a thoracic mass as the etiology of the pericardial effusion. In fact the association of thoracic masses and pericardial effusions has been reported in the literature [17-20]. In our case, the detection on ultrasound of a mass in the left chest in the region of the pulmonary artery prompted us to further evaluate this mass with a chest CT.
The management of a clinically significant pericardial effusion or tamponade usually entails drainage of the excess pericardial fluid by performing an ultrasound guided or ECG guided pericardiocentesis [21]. The pericardial fluid should be sent for routine tests including chemical tests such as total protein or albumin, LDH and glucose microscopic tests such as cell count and cytology, and infectious disease tests such as gram stain and bacterial culture and susceptibility testing. Additional testing of the pericardial fluid for viral serology, for tuberculosis culture and smear for acid fast bacilli, for immunohistochemistry and tumor markers such as carcinoembryonic antigen (CEA) can be done if there is concern for specific coexisting conditions.
Conclusion
Pericardial effusions can be the presenting sign for different organ system diseases. A pericardial effusion might be easily missed as a cause of dyspnea in patients presenting to the ED. The emergency physician should be able to diagnose the presence of a pericardial effusion by picking up suggestive signs present on the ECG and CXR and through confirmation using echocardiography as a bedside tool. The presence of a clinically significant pericardial effusion will mandate immediate treatment to prevent further deterioration in the patient's clinical condition and extensive workup to reach a final diagnosis.
References
- Nachman J, Sather HN, Gaynon PS, Lukens JN, Wolff L, Trigg ME. Augmented Berlin-Frankfurt-Munster therapy abrogates the adverse prognostic significance of slow early response to induction chemotherapy for children and adolescents with acute lymphoblastic leukemia and unfavorable presenting features: a report from the Children's Cancer Group. J ClinOncol. 1997; 15: 2222-2230.
- Leptidis J, Aloizos S, Chlorokostas P, Gourgiotis S. Fatal cardiac tamponade as the first manifestation of acute myeloid leukemia. Am J Emerg Med. 2014; 32: 1294.
- Prihadi E, Hariman C, Janssens L. Acute cardiac tamponade: presenting sign of acute leukaemia. Acta Cardiol. 2011; 66: 251-253.
- Lin E, Boire A, Hemmige V, Husain AN, Sorrentino M, Nathan S, et al. Cardiac tamponade mimicking tuberculous pericarditis as the initial presentation of chronic lymphocytic leukemia in a 58-year-old woman: a case report. J Med Case Rep. 2010; 4: 246.
- Bussani R, De-Giorgio F, Abbate A, Silvestri F. Cardiac metastases. J Clin Pathol. 2007; 60: 27-34.
- Rosenbaum E, Krebs E, Cohen M, Tiliakos A, Derk CT. The spectrum of clinical manifestations, outcome and treatment of pericardial tamponade in patients with systemic lupus erythematosus: a retrospective study and literature review. Lupus. 2009; 18: 608-612.
- Kuvin JT, Harati NA, Pandian NG, Bojar RM, Khabbaz KR. Postoperative cardiac tamponade in the modern surgical era. Ann Thorac Surg. 2002; 74: 1148-1153.
- Shah MB, Biederman RW. Pericardial effusion masquerading as an aortic dissection. Echocardiography. 2011; 28: E16-18.
- Gümrükçüoglu HA, Akyol A, Tuncer M, Günes Y, Begenik H, Akdag S, et al. [Clinical and laboratory features of patients with pericardial effusion]. Turk Kardiyol Dern Ars. 2010; 38: 473-479.
- Ekka M, Ali I, Aggarwal P, Jamshed N. Cardiac tamponade as initial presenting feature of primary hypothyroidism in the ED. Am J Emerg Med. 2014; 32: 683.
- Roy CL, Minor MA, Brookhart MA, Choudhry NK. Does this patient with a pericardial effusion have cardiac tamponade? JAMA. 2007; 297: 1810-1818.
- Guberman BA, Fowler NO, Engel PJ, Gueron M, Allen JM. Cardiac tamponade in medical patients. Circulation. 1981; 64: 633-640.
- Schiavone WA. Cardiac tamponade: 12 pearls in diagnosis and management. Cleve Clin J Med. 2013; 80: 109-116.
- Price AS, Leech SJ, Sierzenski PR. Impending cardiac tamponade: a case report highlighting the value of bedside echocardiography. J Emerg Med. 2006; 30: 415-419.
- Mandavia DP, Hoffner RJ, Mahaney K, Henderson SO. Bedside echocardiography by emergency physicians. Ann Emerg Med. 2001; 38: 377-382.
- Reydel B, Spodick DH. Frequency and significance of chamber collapses during cardiac tamponade. Am Heart J. 1990; 119: 1160-1163.
- Nishi T, Takamori S, Muta F, Yoshiyama K, Iwasaki Y, Shirouzu K. Nonmalignant pericardial effusion associated with thymic cancer. Gen Thorac Cardiovasc Surg. 2010; 58: 239-242.
- Kainuma S, Masai T, Yamauchi T, Takeda K, Ito H, Sawa Y. Primary malignant pericardial mesothelioma presenting as pericardial constriction. Ann Thorac Cardiovasc Surg. 2008; 14: 396-398.
- Nardell EA, Fan D, Shepard JA, Mark EJ. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 22-2004. A 30-year-old woman with a pericardial effusion. N Engl J Med. 2004; 351: 279-287.
- Massari FM, La Marchesina U, Buffa P, Arpesani A, Foresti A. [Benign mediastinal teratoma with pericardial symptoms. The utility of echocardiography in diagnosis]. G Ital Cardiol. 1997; 27: 476-479.
- Cooper JP, Oliver RM, Currie P, Walker JM, Swanton RH. How do the clinical findings in patients with pericardial effusions influence the success of aspiration? Br Heart J. 1995; 73: 351-354.