
Case Report
Austin J Emergency & Crit Care Med. 2015;2(1): 1013.
Do you have an Asymptomatic Surgical Patient with a Massive Venous Thromboembolism? Keep Calm, “don’t touch” and Start Aggressive Anticoagulation!
Colombo J1*, Arena A2, Gamba A3, Codazzi D2 and Langer M1,2
1Department of Pathophysiology and Transplantation, University of Milan, Italy
2Department of Anesthesia, Intensive Care and Palliative Care – Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
3Cardiovascular Department, Manzoni Hospital, Lecco, Italy
*Corresponding author: Jacopo Colombo, Department of Pathophysiology and Transplantation, University of Milan, Via Festa del Perdono 3, Milan, Italy
Received: December 30, 2014; Accepted: February 16,, 2015 Published: February 18, 2015
Abstract
Here we present a case of a 49-year-old man affected by Pseudomyxoma peritonei who experienced extended venous thromboembolism (VTE) 15 days after cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC).
Despite the large sizes of thrombi, the involvement of superior vena cava, right atrium and pulmonary artery, patient was asymptomatic and hemodynamically stable. He had just been subjected to a radical surgery so that we decided to avoid thromboembolectomy on cardio-pulmonary-bypass and to start anticoagulation (continuous intravenous infusion of unfractioned heparin to maintain the APTT in a range of 46-90 seconds).
We monitored sonographically the intracardiac portion of the thrombus: we noticed change in shape and a progressive dimensionality reduction. These findings were confirmed by CT scan, which, on the 14th day of anticoagulant therapy, showed only small residues of the known thrombus. We switched anticoagulant therapy to low-molecular-weight heparins (LMWH) and the patient was moved to his surgical ward.
Pre-demission CT scan showed no more evidence of VTE.
We can conclude that medical treatment was the right choice because aggressive anticoagulant therapy allowed regression and disappearance of thrombus without any complication.
Keywords: Venous thromboembolism; Anticoagulant therapy; Thromboembolectomy; Pseudomyxoma peritonei; Citoreduction; HIPEC
Case Presentation
A 49-year-old man, affected by Pseudomyxoma peritonei, was scheduled for cytoreductive surgery (CRS), involving peritonectomy and multivisceral resections with intra-operative hyperthermic intraperitoneal chemotherapy (HIPEC).
The patient was in excellent conditions; routine preoperative investigations showed no abnormalities. Postoperative monitoring in intensive care unit was planned for length and invasiveness of surgery.
Balanced anaesthesia was performed without complication. A central venous catheter (CVC) was placed to allow perioperative drug administration.
After uncomplicated surgery (CRS and HIPEC with cisplatin 150 mg plus mitomycin 25 mg, perfusion time 60 minutes at 42,5oC) and anaesthesia, the patient was admitted to the intensive care unit (ICU). He was discharged from the ICU on postoperative day 1 pain-free and following an uneventful stay.
No abnormalities in the routine postoperative blood tests were observed.
For the following two weeks he kept to the department protocol for VTE prophylaxis (one-daily-dose of subcutaneous Nadroparin Calcium 0,4 mL) and post-surgical rehabilitation without any complications.
On the 15th postoperative day, the patient became pyretic, shivered and inflammatory markers rose (C-reactive protein 271 mg × L-1 and leukocyte count 15.310 × 109 × L-1). No variations in hemodinamic and respiratory parameters were noted. Blood culture samples were taken and the patient was started at broad-spectrum antibiotics (piperacillin- tazobactam, teicoplanin, fluconazole).
Surgeons required an abdomen CT scan with iv contrast for suspected abdominal abscess. No intrabdominal problem was noted but cranial slices revealed thromboembolism in right pulmonary artery. CT scan of thorax with iv contrast was done to see the extent of the thrombus and showed thromboembolism in right pulmonary artery and a large thrombus in superior vena cava extending into the right atrium.
Lower limbs’ ecocolordoppler didn’t show any sign of deep venous thrombosis (DVT).
Surgeons involved intensivists for case management: we consulted three cardiac surgeons; two of them laid the indication for surgical embolectomy on cardio-pulmonary-bypass (CPB).
Patient was asymptomatic, hemodynamically stable and he had just been subjected to a radical surgery so that we decided to avoid thromboembolectomy and to embrace the more conservative policy given by the third cardiac surgeon, the medical therapy.
The patient was moved to ICU to start continuous intravenous infusion of unfractioned heparin (UFH). Treatment was initiated with a bolus injection of 80IU/Kg, followed by continuous infusion of 18IU/kg/hr.
Serial blood samples were planned to monitor anticoagulation (activated Partial Thromboplastin Time (APTT) and antithrombin III values) and continuous infusion was adjusted to maintain aPTT in a range of 46-90 seconds (Figure 1). Few days later blood-cultures became positive for Staphylococcus Epidermidis so that a targeted antibiotic therapy with Daptomycin was started.
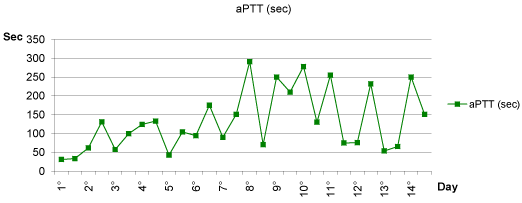
Figure 1: aPPT values , expressed in seconds, measured every 12 hours
from the first day of UFH continuous infusion up to the last day of endovenous
treatment (day 14). We adjusted continuous infusion to reach the target aPTT
range of 46-90 seconds.
We performed serial echocardiographies to monitor intracardiac thrombus: we noticed a change in shape and a progressive dimensionality reduction (Table 1).
EXAM
THROMBUS DESCRIPTION
MEASUREMENTS
DAY of HEPARIN THERAPY
CT-scan
thromboembolism in right pulmonary artery and large thrombus in superior vena cava extending into the right atrium
-----
0o : continuous intravenous infusion
of UFH started after these exams
US
multilobulated mass in right atrium, with irregular profile and movable parts
25mm X 46mm
US
oblong multilobulated mass in right atrium, with movable parts extending until tricuspid valve surface
22mm X 54mm
4° UFH
US
no more presence of distal filamentous thrombus’ part extending until tricuspid valve surface
17mm X 45mm
7° UFH
CT-scan
residual small size thromboembolism in right pulmonary artery,superior cava and right atrium
-----
14° UFH
US
smaller interatrial mass with less fluctuating parts
6mm X 23mm
7° LMWH
CT-scan
residual small size thromboembolism in right pulmonary artery, no more evidence of VTE in superior cava and right atrium
-----
14° LMWH
Table 1: VTE evolution assessed by serial echocardiographies and thorax CT-scan. 28 days of anticoagulation therapy have led to change in shape and a progressive dimensionality reduction of thrombus..
CT performed after fourteen days of UFH continuous infusion confirmed ultrasonographic findings and showed dimensional reduction of thrombus also in right pulmonary artery and superior cava (Figure 1). On the 14th day of anticoagulant therapy without complication, we switched UFH to low-molecular-weight heparins (LMWH, Nadroparine Calcium 0,8 mL twice a day) and the patient was moved to his surgical ward.
The following hospital stay was uneventful and after two weeks the patient was discharge at home.
scan performed before discharge showed residual small size thromboembolism in right pulmonary artery and no more evidence of VTE in superior cava and right atrium (Table 1, Figure 2).
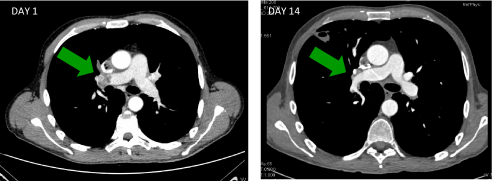
Figure 2: CT scan before and after 14 days of anticoagulation: thromboembolism in right pulmonary artery reduced significantly.
Dismissal’s prescriptions were to continue Nadroparine Calcium 0,8 mL twice a day for 6 months and to come in outpatient infectious diseases daily for four weeks to continue the administration of Daptomycin.
He finished antibiotic cycle and LMWH without having any side effects.
Discussion
Pseudomyxoma peritonei (PMP) is a rare disease (incidence 1/1.000.000inhab/year) that is characterized by the production and accumulation of copious amount of mucinous ascites within the peritoneal cavity [1].
Patients with PMP are treated with CRS and HIPEC. CRS include peritonectomy at the anatomical sites where there is visible evidence of disease to ensure all macroscopic tumors are removed or, if not possible, leaving no nodules bigger than 5 mm. In sites where there is major tumor involvement of visceral organs, resections are performed [2,3].
At the end of surgical procedure, but before intestinal anastomosis or repair of seromuscular tears, HIPEC is performed for 60 -90 minutes using an open abdomen technique with high dose chemotherapeutic drugs at 42oC, to improve drug distribution, to erase microscopic residual tumour. The 5-year survival results of debulking surgery from large retrospective series ranged from 55–75% [4,5].
CRS is a very long, destructive and bloody surgery, which leads to peritoneum disepithelization.
This surgical trauma, associated with hyperthermia produces complex local and systemic responses, comparable to that of major burns. The local inflammatory response results in vasodilatation and an increase in vascular permeability with extravasation of fluid and plasma protein and oedema formation. Hand in hand with the local response a systemic inflammatory response syndrome (SIRS) develops, which leads to hemodynamic alterations and to activation of coagulation.
It’s clear that, in these patients, risk of venous thromboembolism (VTE) is tightly enhanced in the perioperative period. In a previous report, Lanuke et al. reported that 10% of patients who had cytoreductive surgery and hyperthermic intraperitoneal chemotherapy experienced VTE [6].
VTE, which is defined as the presence of clotted blood in a vein, right cardiac chamber or in the pulmonary arterial tree, is quite common in general population (incidence 1.92/1,000inhab/year).
Patients with cancer have at least a six fold increased risk of VTE and VTE is the second most common cause of death in cancer patients. This is a reflection of the hypercoagulable state associated with malignancy, combined with the thrombogenic effects of cancer therapies [7]. Mucinous tumours of the gastrointestinal tract are associated with higher thromboembolic risk compared to other solid tumour [6].
Patients who undergo surgery to treat a cancer have a risk two-three times greater to present VTE as compared with general population. In the same way, chemotherapy increases VTE risk of three-six times [7].
Moreover the placement of a CVC may be the breeding ground for the occurrence of thrombotic events. The reported incidence of CVC-associated thrombosis varies widely in the literature and it ranges from 0 to 20% in cancer patients [7].
We must emphasize that, in addition to the risk factors listed above for which prophylaxis was designed, the patient had a Staphylococcus Epidermidis sepsis.
It’s well known that sepsis is associated with haemostatic changes: the systemic inflammatory response to the infectious agent that causes up-regulation of procoagulant pathways, down-regulation of physiological anticoagulants and suppression of fibrinolysis [8].
In addition, the presence of bacteremia and definite intracardiac emboli are two Duke diagnostic criteria of infective endocarditis, which is itself an additional risk factor for VTE [9].
Summing up our patients had many risk factors for VTE: cancer, surgery, high dose chemotherapy, CVC, sepsis, and infective endocarditis.
As regards the therapy undertaken to treat VTE, the high risk of bleeding, given the recent surgical intervention, influenced our choice. We preferred medical therapy which was longer and was less likely to succeed than surgical embolectomy because it was the only therapy which, in case of a massive bleeding from cruentated abdominal surfaces, would allow us a rapid ricoagulation. Abdominal bleeding during CBP would have been difficult to control. Furthermore surgical technique for removal of thrombi was not clear and probably it would not remove all intravascular thromboembolic formations, requiring a postoperative anticoagulation. It would be performed in a septic patient, condition which itself increases the perioperative risk. Last but not least, surgical embolectomy would have required the transfer of this patient to another hospital, where, due to the inexperience with oncology patients who recently underwent CRS and HIPEC, management would be more difficult.
We decided to avoid thrombolysis with simultaneous anticoagulant administration, which could lead to a more rapid reduction of the thrombus, because, according to the “Pulmonary embolism severity index” the patient was “low risk”: he was hemodynamically stable, he had no problems with gas exchange and the state of consciousness was not altered. In Figure 3 we summarize pro and cons of possible therapies of VTE in surgical patients.

Figure 3: Pro and cons of possible therapies.
Ex post, medical therapy alone was the best choice: it allowed thrombi to regress and avoided all risks related to thromboembolectomy on CPB. We can therefore conclude that aggressive anticoagulation is the first therapeutic choice in asymptomatic surgical patient with a massive venous thromboembolism.
There is another take home message: in very high risk population recommended prophylaxis is not enough. Prophylaxis is well known to be the most appropriate strategy to reduce VTE related morbidity and mortality.
In this case, however, one-daily-dose of LMWH was sufficient to prevent DVT but couldn’t avoid thromboembolism in right pulmonary artery and a large veno-atrial thrombus.
In patients with multiple risk factors that overlap we have to optimise and enhance the antithrombotic prophylaxis and make routine active screening even when they are clinically silent.
References
- Mukherjee A, Parvaiz A, Cecil TD, Moran BJ. Pseudomyxoma peritonei usually originates from the appendix: a review of the evidence. Eur J Gynaecol Oncol. 2004; 25: 411-414.
- Jacquet P, Sugarbaker PH. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res. 1996; 15: 49–58.
- Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995; 221: 29-42.
- Gough DB, Donohue JH, Schutt AJ, Gonchoroff N, Goellner JR, Wilson TO, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg. 1994; 219: 112-119.
- Miner TJ, Shia J, Jaques DP, Klimstra DS, Brennan MF, Coit DG. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg. 2005; 241: 300-308.
- Lanuke K, Mack LA, Temple WJ. A prospective evaluation of venous thromboembolism in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Can Chir. 2009; 52: 18 – 22
- Khorana AA. Cancer and coagulation. Am J Hematol. 2012; 87: S82-87.
- Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res. 2012; 129: 290-295.
- Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000; 30: 633-638.