
Special Article - Burns
Austin J Emergency & Crit Care Med. 2015;2(4): 1026.
Hand Burns
Bailey JK¹*, Zomerlei TA², Coffey R¹ and Jones LM¹
¹Department of Surgery, Ohio State University, USA
²Department of Plastic Surgery, Ohio State University, USA
*Corresponding author: Bailey JK, Department of Surgery, The Ohio State University, N748 Doan Hall, 410W, 10th Avenue, Columbus, OH 43210, USA,
Received: May 13, 2015; Accepted: July 06, 2015; Published: July 10, 2015
Abstract
The incidence of burns to the hand may be much more common than reported in medical literature. The hand is such an important means of interacting and exploring our environment that these burns need to be treated carefully. Fortunately, most burns are relatively minor, so that only more severe injuries present for evaluation and treatment. Optimal outcomes require a coordinated effort early on in the course of recovery for the burn patient.
Keywords: Hand burns; Management; Blisters; Escharotomy
Introduction
The hands and upper extremity are involved with thermal injury about forty percent of the time in published series, but this number is probably even higher if smaller burns that present to primary care physicians and clinics are included [1]. Fortunately, most of these injuries are self-limited and typically cared for at home, or heal uneventfully with only basic care. However, more severe injury (either from extent of local injury or associated injuries) can lead to substantial disability and require subspecialty care [2,3]. As with other mechanisms of hand injury, ideal care requires a team approach. Optimal outcome requires an early focus on maintaining as much mobility and function as possible, rather than allowing disability to develop.
Anatomy and pathophysiology
Thermal injury to the skin is proportional to the intensity of the noxious insult (how high the temperature and for how long) and ameliorated by protective factors such as the presence of protective garments and the relative thickness of the skin [4]. Injury can be first noted by the presence of simple hyperemia or 1st degree burn. This is analogous to uncomplicated sunburn which does not blister. Blistering or separation at the dermal-epidermal interface is a sign of deeper injury, at least a 2nd degree burn. Epithelial cells are also located in hair follicles and sweat glands. These epithelial cells function as a reservoir that can repopulate the injured surface of a burn. The relative density of these reservoirs as well as their orientation (that is how deeply they extend into the dermis) can both contribute to the likelihood of an area of burn injury healing (Figure 1). The repopulation of the epidermis is thought to modulate healing and curb inflammation with concomitantly less scar production [5]. If enough of the reservoir fails to survive the injury (either from the initial insult or from poor wound care), then the wound will close secondarily with granulation tissue (that is some degree of scar formation). When the skilled examiner inspects the initial injury, the main focus of description is the extent of injury (that is the size of burn) and predicted chance of healing (that is the depth of burn). From a burn surgeon's perspective, there are partial-thickness injuries and full-thickness injuries. Partial-thickness injuries are first-degree burns and second-degree burns, cases in which there is a sufficient reservoir of epithelial cells to allow healing to progress over 2-3 weeks. Full-thickness burns are 3rd degree burns or very deep second-degree burns. In other words, there may be viable dermis, and perhaps even a few scatter reservoirs of epithelial cells, but the wound cannot be expected, to heal by simple migration of epithelial cells (Figure 2). Unfortunately, this classification system sometimes includes descriptions such as superficial partial thickness and deep partial thickness, which may be confusing.
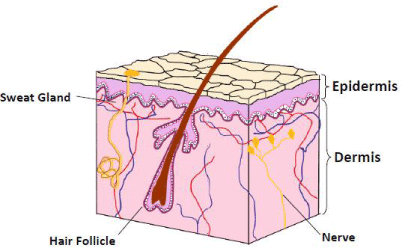
Figure 1: Low detail depiction of skin highlighting presence of epithelial cells
along hair follicles and some sweat glands which can function of reservoir of
epithelial cells to grow back over surface of exposed dermal layer after burn
injury. (Modified with permission).
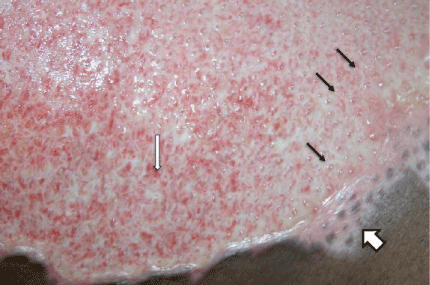
Figure 2: Grease scald showing good demarcation at 14 days post burn.
Note difference between areas of epithelial growth (so called “epi-pearls”
noted by small black arrows) and granulation tissue formation (with capillary
tufts demarcated by narrow white arrow). Finally, an area that quickly healed
has evidence of melanocyte repopulation (wide white arrow).
Identification of wounds expected to heal secondarily is important for best outcomes. Allowing these wounds to granulate and heal by a process, essentially, of scar formation can lead to significant dysfunction. Figure 3 demonstrates the effect of a scald injury on the dorsal surface of the hand and wrist of an adult allowed to heal by secondary intention. Eventually, the wounds did close without skin grafting, but the resulting contracture forced the hand into a poorly functioning position. Early surgical grafting is used to avoid this complication (loss of function) of spontaneously healing full thickness injuries.
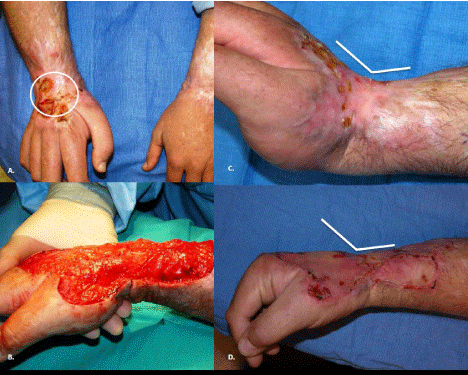
Figure 3: Results of untreated scald injury to dorsum of hand and wrist. A.
Note open wound (over one year since injury). B. Excision of relatively small
area of contracted scar reveals closer estimation of original burn size. C.
Contracture of scar limited range of motion with fixed extension position of
wrist (note angle). D. After excision of scar and application of skin graft (2
weeks) able to obtain neutral position of wrist.
Initial wound care
Acknowledging, that burns to the hand can be either an isolated injury or only one of a complex multi-trauma patient’s injured organs, the present discussion will systematically describe our approach to care of the burn. Initial assessment must include appropriate assessment of pertinent history and concomitant injuries and this is best applied along guidelines described in Advanced Trauma Life Support courses [6]. Isolated burn wounds of the hand may be cooled initially with a moistened dressing or cool tap water. If there is suspicion of chemical injury, then the offending agent should be removed and the skin irrigated with tap water [7]. Simple irrigation is the appropriate initial treatment for almost all offending agents. One notable exception is exposure to hydrofluoric acid (Figure 4). First line treatment also includes topical calcium, and should this fail consideration given to intra-arterial infusion of calcium [8].
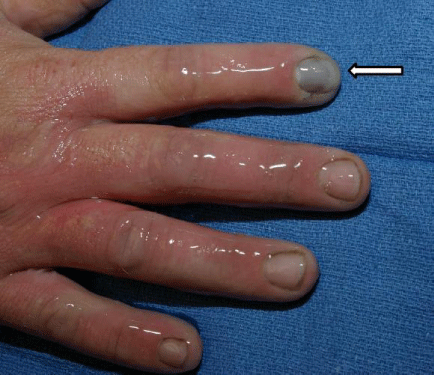
Figure 4: Example of hydrofluoric acid burn. Moist appearance to hand is
from application of aqueous gel compound of calcium gluconate. Arrow,
points to early discoloration from injury to nail bed. Removal of nails may be
necessary in cases of chemical exposure.
In addition to the skin assessment, a thorough examination of both hands should include examination of neuromuscular function and adequacy of perfusion. Circumferential burns should be noted, especially evidence of full-thickness injury. Normal skin is elastic and allows for a change in volume with edema formation. When skin sustains a full thickness thermal injury, it becomes leathery and less elastic. As edema accumulates, there is limited change in volume, and instead pressure increases. The inelastic/nonviable skin (eschar) becomes a restrictive layer analogous, though superficial, to the fascial layer of muscle. This condition of increased sub-eschar pressure is readily amenable to escharotomy as appreciated by Pruitt et al. [9]. Of special note, although presented in juxtaposition to initial wound care, the need for escharotomy slowly develops over the course of the resuscitation. Escharotomy is much less likely at initial presentation and in cases of uncomplicated local injury. Secondly, successful completion of escharotomy does not eliminate the potential need for fasciotomy, especially in cases of deep burn, electrical injury, and large volume resuscitations associated with large burns. When monitoring for potential development of compartment syndrome (be it from inelastic circumferential eschar or from the fascia), the need for a high index of suspicion cannot be overstated. When a conscious patient is queried directly the patient can report and distinguish the deeper "muscle pain" of developing ischemia from the pain in the skin. They can also be asked to monitor for paresthesia. Given an isolated injury not requiring resuscitation in combination with a reliable patient and availability of follow-up in coordination with a Burn Center, it is safe to manage these injuries as an outpatient. Not every circumferential burn of the hand needs inpatient monitoring as seen in Figure 5. If there is any doubt as to the patient’s ability to reliably communicate the onset of pain or paresthesia, due to an unreliable patient or unreliable physical exam, the compartment pressures should be measured. This can be done simply by setting up as if one were to transduce a central venous pressure (Figure 6). Attach an 18-gauge needle to the distal end of the pressure tubing, and then hold the needle tip at the level of the compartment to be measured. Zero the reading with the needle (which is open to the air) held at the same level on the vertical plane as the intended insertion point. Then the needle is advanced into the compartment and withdrawn 1-2 mm before taking a reading. Measuring pressures greater than or equal to 30 mmHg can indicate the need for escharotomies. This number is deserving of routine treatment as, by definition, eschar is dead skin that is so severely damaged it will need to be grafted. This makes any resulting scar from the incision of the skin moot.
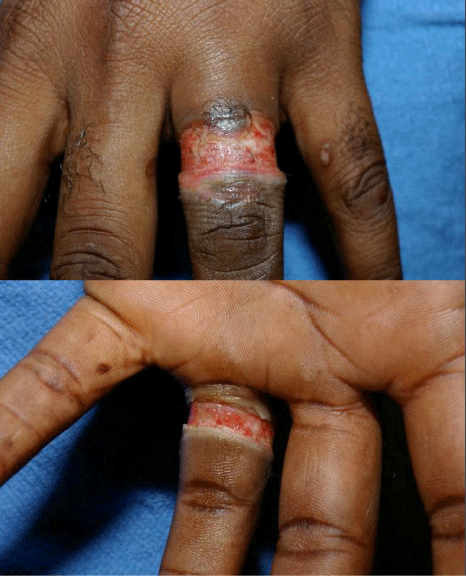
Figure 5: Approximately 2 weeks after electrical burn when wedding band in
contact with household current. No escharotomy was necessary in this case.
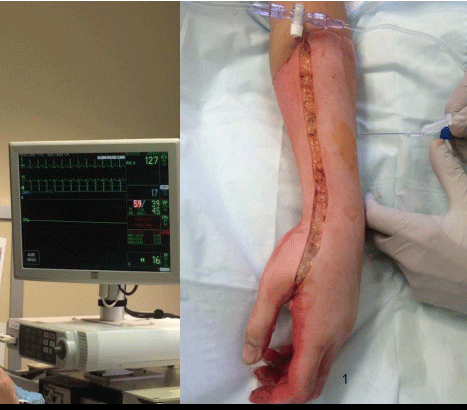
Figure 6: Use of bedside pressure monitoring. The patient’s nurse has set
up as if to measure Central Venous Pressure (CVP), which is seen to be 17
mm Hg on screen. Needle is connected to tubing and zeroed at the same
vertical level the clinician intents to measure the compartment pressure.
Once zeroed, the needle is advanced into the compartment then carefully
withdrawn one to two millimeters before reading the measurement.
The treatment of blisters - to debride or not debride is a subject of some reasonable discussion [10,11]. Proponents of leaving blisters intact, highlight the fact that the intact blisters may act as biologic dressings and decrease the amount of pain. However, debriding the blisters may aid in healing with a resultant decrease in inflammatory mediators. This also allows for increased range of motion and may decrease the chance of colonization and infection [10]. This is of special concern in cases of immunocompromised or unreliable patients (Figure 7).
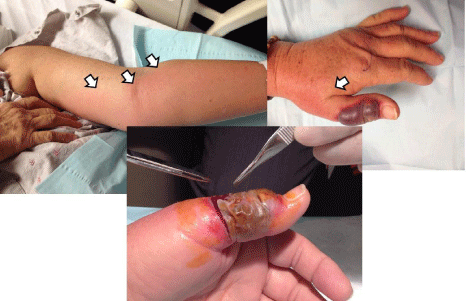
Figure 7: Illustrative case of potential hazard of leaving blister unroofed.
Elderly patient undergoing chemotherapy had minor burn from cooking to
dorsum of left thumb. Over two to three days, became more sanguineous
in appearance and presented with ascending lymphangitis (arrows show
border). Blister unroofed and gram stain and cultures showed infection due
to a methicillin-sensitive Staphylococcus species.
Burned skin may be sterilized initially by the intense heat from the injury. However, the skin is rapidly recolonized by the patient's own flora. This colonization is not an active infection and routine use of systemic antibiotics is of no routine value. However, topical antibiotics can aid in controlling colonization. Popular topical therapies for burn wounds include three approaches: 1) Silver sulfadiazine 2) Bacitracin 3) Silver-based dressing. Silver sulfadiazine is one of the most commonly used topical agents for burns. It has a wide spectrum of antimicrobial activity, is soothing and generally stocked in most emergency departments. It should be used with caution in the last trimester or for infants up to 2 months of age and in case of concerns of G6 PD deficiency [12]. Bacitracin is another topical ointment manufactured from growth of the bacteria Bacillus subtilis, Tracy 1. It is also effective against gram-negative as well as gram-positive bacteria, although it lacks activity against fungus. There is some evidence that may allow for more rapid in vitro epithelial growth and it comes in anophthalmic ointment, which can be applied to burns where there is a risk of accidental contact with the patient's eye [13]. For the primary care physician it is worthwhile to remember that patients can develop a contact dermatitis from bacitracin, neomycin, and or polymixin B. This can create a much more troubling skin condition than the original burn. Polysporin® contains both polymixin B and neomycin. Neosporin® contains all three antibiotics–bacitracin, neomycin, polymixin B. It is for this reason (minimizing risk of dermatitis), that we recommend the simplified treatment of using the simplest antibiotic possible. In the cases of small burns complicated by a more impressive dermatitis, seen by the authors, the skin condition from the treatment was truly worse than the “cure” (Figure 8). There are also a multitude of dressings available, which release ionized silver in a controlled and sustained fashion [14]. These dressings can be left in place over partial-thickness burns for up to 1 week, thus decreasing the need for supportive care at home as well as decreasing the pain of wound care. This is advantageous to managing complex wounds that are expected to heal on an outpatient basis.
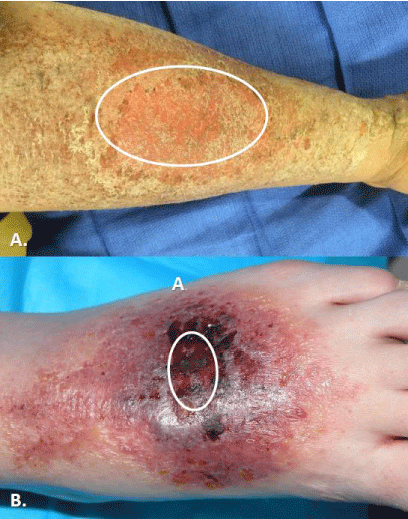
Figure 8: Cases of contact dermatitis as presenting complaint of minor burns.
In both photos, area of original burn is highlighted by circle. A. Case of minor
grease scald. B. Case of friction from rubbing on rug.
When using topical ointments is worth noting that a very convenient dressing strategy has been described decreasing the challenge of teaching a complex weaving of gauze around multiple burned fingers. As seen in (Figure 9), a non-sterile exam glove can be placed over a burned hand after the antimicrobial agent of choice has been applied [15,16]. The patient can be given a handful of gloves to be reapplied after each cleaning and application of ointment. Finally, the most common issue of burns that we encounter is oversight of the fact that full-thickness burns are tetanus prone wounds so current immunization status must be assured [17].
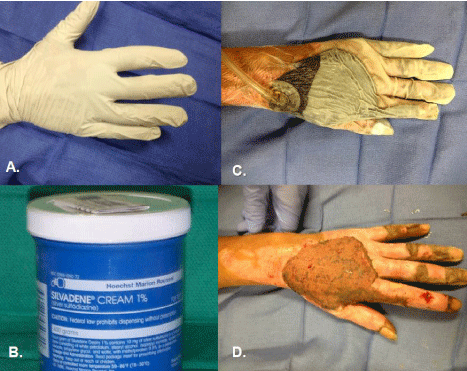
Figure 9: Applications of both sterile and non-sterile gloves in course of care
of a burned hand. A. A non-sterile exam glove may be used over the applied
topical agent of choice (B.) as the dressing. Patients can then easily remove
for washing and reapply with minimal to no assistance. C. We, as others,
have also found sterile gloved a useful adjunct to apply vacuum-assisted
closure device to hand burns, such as these grafts (D.).
Collaboration with occupational therapy and a mind towards rehabilitation needs is essential very early on in the course of care. A therapist’s involvement can aid with fabrication and application of orthotics, preservation of range of motion, and edema management. Because of the pain and edema of the injured hand, patients will position their fingers at such an angle that the metacarpal-phalangeal joints will be held with the joint ligaments at less than maximal tension. This malposition then allows for shortening or tightening of the ligaments of the joints of the finger and can occur in as little as a week (especially in older adults). The ligamentous tightness can require weeks of therapy to recover normal range of motion (consider the challenge of getting the average adult to try to do the splits). Edema can be minimized by elevating the hand at or above the level of their heart which will also ease the pain. In addition, a light compressive dressing can be applied to encourage reduction of edema formation initially. Later strategies include the use of Coban™ or compression gloves. that is often referred to as a reconstructive ladder. Consideration of the appropriateness of allowing time for spontaneous healing is the initial step. In keeping with the analogy of climbing a ladder, each rung implies additional difficulty or technical challenge, but also a progressive accumulation of risk. So, at one extreme a patient who can heal without any surgery and achieve normal form and function should be allowed to heal. If, by chance, the initial plan does not work, and the entire burn does not heal by reconstitution of normal skin, then a second option can be considered with no loss from the initial "failure". Contrast this with the most complex option. A free tissue transfer (or flap) failure means not only lack of wound closure, but also additional tissue loss with failure of the flap. So, it is this riskbenefit assessment that the surgeon works through with the patient in approaching treatment options.
All operative interventions begin with excision of nonviable tissue. Excision is typically done tangentially whereby tissue is shaved off by layers thousandths of an inch in thickness. Successive layers are removed until uniform tissue viability is assured. This process can lead to significant blood loss - as much as ten percent of the total blood volume per percent area excised, or upwards of 400ml/per percent excised [18]. However, the use of tourniquet, and infusion of crystalloid solutions with epinephrine into the areas to be excised can significantly decrease the amount of blood loss – between one and two percent of total blood volume per percent excised [19].
For very small burns, primary closure can be contemplated. This can be performed on the dorsum of the hand, but the digits and palmar surface typically do not afford enough tissues laxity to permit this option. If this is the case then the next option would typically be use of skin graft.
Full-thickness skin grafts offer the advantage of decreased donor site pain since the donor sites may be closed primarily with non-absorbable suture. In addition, full-thickness skin grafts do not contract with time and are generally more pliable. However, fullthickness skin graft allow for transplantation of hair. The hair may be troublesome if transplanted to the dorsum of the finger in a female or the palmar surface. Secondly, there is a limit to the size of skin graft, which can be taken (Figure 10). Split-thickness skin graft offers the option to cover the entire surface of both hands if necessary (Figure 11). Although the supply is greater, the grafts tend to contract more, which can make proper therapy and aftercare more important and challenging [20]. In addition, there is more donor site morbidity as partial-thickness donor site areas are more painful, and the skin tone generally undergoes a permanent change. Finally, choice of donor site should take into account the fact that skin color is slightly different in different regions of the body-so as to minimize cosmetic contrast.
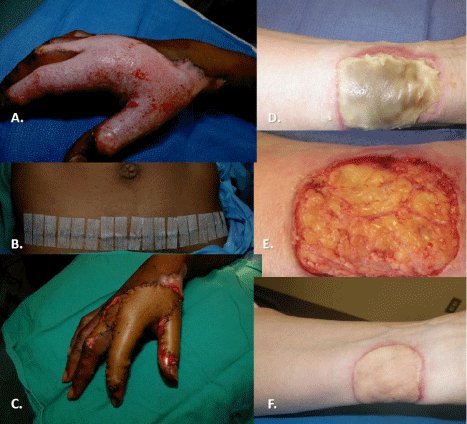
Figure 10: A. Full-thickness scald of the hand treated with a full-thickness
skin graft from the infra-abdominal crease line (B.). Even with such an
apparently long incision, there are limits to the size of ellipse of skin that
can be taken, and this limits the size of injuries that can be treated (C.).
Another case of a contact burn to the wrist (D.) required excision down to
and including some subcutaneous fat was covered with a full-thickness graft
from the lateral groin area of the patient. F. The graft healed well, but note the
difference in skin tones (more yellow) between skin from different anatomic
location on same patient.
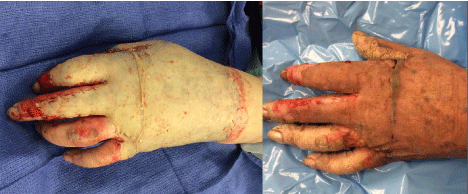
Figure 11: Split-thickness graft to grease scald burn shortly after application
in the operating room (image on left). By one week (photo on right), there
is evidence of only small hematomas, and generally excellent “take”. Note,
it is much easier to cover a large surface area with split-thickness skin graft.
Before leaving the discussion on grafting, it is worth mentioning the other options-the use of xenograft, allograft, and Integra®. Xenograft, porcine dermis, offers off the shelf convenience though limited durability. In our opinion, it is best used for temporary coverage when allograft is not available and needed for no more than one week. Allograft, cadaver skin, can be used for intermediate term coverage up to 2-3 weeks, but has increased cost and a hypothetical risk of transmission of human viral pathogens. It is used when the condition of the donor site or recipient site is less than ideal and the surgeon reasonably expects improvement with time. Examples include an unstable patient or a malnourished patient who may be at risk of a donor site becoming a non-healing wound. Finally, Integra® is a permanent dermal substitute in which the patient's own cells and blood vessels populate a deeper “honeycomb” layer of the product to produce a neo-dermis. Once this migration is complete (approximately 3 weeks) then the superficial layer of silicone is carefully peeled off and a split-thickness skin graft applied. Integra® is often useful for coverage of deep or vital structures such as tendons (Figure 12).
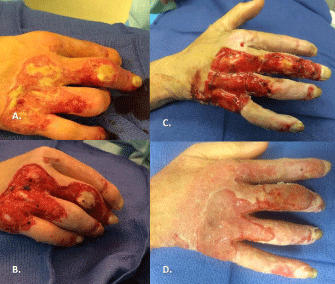
Figure 12: A. More challenging burn scalds to hand of patient with very thin
dorsal skin due to chronic disease. B. At time of excision, patient found to
have multiple dorsal tendons exposed. C. Integra was applied and is seen
here after two weeks of maturation. D. A split-thickness skin graft was applied
and is see here three weeks after skin grafting.
Local flaps can offer permanent coverage of burns especially small but deep burns over vital structures, especially on the dorsum of the finger or thumb. The palmar skin is anchored to the bone, which gives greater stability than but does limit flexibility as a result; there are relatively limited numbers of options for local flaps to the palmar defects. The tips of digits skin from the palmar side of the distal phalanx may be advanced by about half a centimeter toward the dorsal skin. Dorsal skin may also be rotated volar to cover deficit, a cross-finger flap. The skin bridge is subsequently divided in about 2 weeks. There are also a host of options for soft tissue coverage tissue of the hand using more remotely located tissue (rotational and free flaps). However, further exploration of the details of the surgical decision-making is beyond the scope and intent of this manuscript.
Summary
Burns to the hand and wrist present unique challenges for care. Initial assessment of burn depth with the formulation of a multidisciplinary plan of care including wound care and a surgical plan. Early consultation with the occupational therapist is key for the best functional outcomes.
References
- Chen SH, Chen YC, Chen TJ, Ma H. Epidemiology of burns in Taiwan: a nationwide report including inpatients and outpatients. Burns. 2014; 40: 1397-1405.
- Dodd AR, Nelson-Mooney K, Greenhalgh DG, Beckett LA, Li Y, Palmieri TL. The effect of hand burns on quality of life in children. J Burn Care Res. 2010; 31: 414-422.
- Lin SY, Chang JK, Chen PC, Mao HF. Hand function measures for burn patients: a literature review. Burns. 2013; 39: 16-23.
- Moritz AR, Henriques FC. Studies of Thermal Injury: II. The Relative Importance of Time and Surface Temperature in the Causation of Cutaneous Burns. Am J Pathol. 1947; 23: 695-720.
- Brown DL, Kao WW, Greenhalgh DG. Apoptosis down-regulates inflammation under the advancing epithelial wound edge: delayed patterns in diabetes and improvement with topical growth factors. Surgery. 1997; 121: 372-380.
- Trauma TACoSCo: Advanced Trauma Life Support (ATLS) for Doctors. 8th edn. Chicago, IL. 2008.
- Andrews K, Mowlavi A, Milner SM. The treatment of alkaline burns of the skin by neutralization. Plast Reconstr Surg. 2003; 111: 1918-1921.
- Robinson EP, Chhabra AB. Hand chemical burns. J Hand Surg Am. 2015; 40: 605-612.
- Pruitt BA Jr, Dowling JA, Moncrief JA. Escharotomy in early burn care. Arch Surg. 1968; 96: 502-507.
- Rockwell WB, Ehrlich HP. Should burn blister fluid be evacuated? J Burn Care Rehabil. 1990; 11: 93-95.
- duKamp A. Deroofing minor burn blisters--what is the evidence? Accid Emerg Nurs. 2001; 9: 217-221.
- Eldad A, Neuman A, Weinberg A, Benmeir P, Rotem M, Wexler MR. Silver sulphadiazine-induced haemolytic anaemia in a glucose-6-phosphate dehydrogenase-deficient burn patient. Burns. 1991; 17: 430-432.
- Smoot EC 3rd, Kucan JO, Roth A, Mody N, Debs N. In vitro toxicity testing for antibacterials against human keratinocytes. Plast Reconstr Surg. 1991; 87: 917-924.
- Barillo DJ, Marx DE. Silver in medicine: a brief history BC 335 to present. Burns. 2014; 40: S3-8.
- Mashiko T, Ohnishi F, Oka A, Kawauchi T, Shiokawa I, Yamakawa T, et al. Usefulness of surgical glove dressing: a novel technique for skin graft fixation after hand burns. J Plast Reconstr Aesthet Surg. 2013; 66: 1304-1306.
- Alexander MJ. Surgical glove treatment for hand burns. JACEP. 1977; 6: 69.
- Culbertson TA, Kalliainen LK, Buchele BA. Tetanus and the plastic surgeon. Ann Plast Surg. 2004; 53: 162-165.
- Steadman PB, Pegg SP. A quantitative assessment of blood loss in burn wound excision and grafting. Burns. 1992; 18: 490-491.
- Cartotto R, Musgrave MA, Beveridge M, Fish J, Gomez M. Minimizing blood loss in burn surgery. J Trauma. 2000; 49: 1034-1039.
- Corps BV. The effect of graft thickness, donor site and graft bed on graft shrinkage in the hooded rat. Br J Plast Surg. 1969; 22: 125-133.
- Hollinger JO. An introduction to biomaterials. 2nd edn. Boca Raton, FL: CRC Press/Taylor & Francis. 2012.