
Special Article - Burns
Austin J Emergency & Crit Care Med. 2016; 3(1): 1043.
Prediction, Identification, and Initial Treatment Guide for Scald Injuries
Bourdon RT¹, Nelson-Cheeseman BB² and Abraham JP²*
¹Emergency Department, Regions Hospital, USA
²School of Engineering, University of St. Thomas, USA
*Corresponding author: Abraham JP, School of Engineering, University of St. Thomas, 2115 Summit Ave, St. Paul, MN 55105-1079, USA
Received: May 13, 2016; Accepted: June 21, 2016; Published: June 24, 2016
Abstract
The prediction and treatment of hot-liquid scald injuries is complicated by the complex heat transfer within living tissue, incomplete information associated with scald incidents, and inherent limitations with accurate injury-depth estimation. To provide some guidance for the prediction, identification, and initial treatment, a single comprehensive resource is valuable. This review addresses these three issues. With regard to prediction, use is made of state-of-the-art hot-liquid scald information from the scientific literature. For both identification and treatment, the scientific literature and accepted standards of medical care are elucidated. The primary focus here is on hot-beverage spills because of their frequency; however, the results can be used for other hot-liquid scalding scenarios. Recommendations are made to avoid serious burn injuries and to rapidly treat injuries that occur
Keywords: Scald injuries; Medical care; Burn
Introduction
Burns are a very common form of injury; they occur in residences, workplaces, and public venues. While burns can be generated by a wide variety of causes, very often it is by exposure of skin to hot liquids. These burns, typically referred to as scald injuries; will be the focus of this manuscript.
Accurate assessment of burn depth, burn location, and Total Body Surface Area (TBSA) burned are critical. These three factors direct the management and treatment of burn patients in three main ways. First, each factor informs the decision of whether or not to transfer a patient to a burn center [1-3]. Second, by estimating TBSA burned, clinicians can estimate intravenous (IV) crystalloid fluid requirements over the first 24 to 48 hours [1-4] after a burn injury. Lastly, the depth of a burn predicts whether the injury will likely need excision and grafting [1,3,5]. While there are methods to estimate burn surface area, it is often more difficult to accurately identify burn depth immediately after injury [3,6-9]. This is significant as burn depth largely determines subsequent care [1-3,5]. To help address this uncertainty, predictions of burn depth based on common hot liquid factors will be presented.
Classification of Burns
Burns are classified based on the depth of dermal injury. A commonly utilized classification is the degree categorization. Using this metric, first-degree burns refer to those that are limited to the outmost layer of skin, the epidermis. Second-degree burns pass through the epidermis and into the underlying dermis. The dermis contains many skin structures such as hair follicles, sweat glands, capillaries, and nerves. Second-degree burns can be further classified into superficial partial thickness burns and deep partial-thickness burns. Third-degree burns are those that pass all the way through both the epidermis and dermis. They may enter into the third major layer, the hypodermis, which is primarily composed of fat and connective tissue. Lastly, fourth-degree burns are broadly categorized as those that pass deep into the subcutaneous tissue; they may extend into or through underlying muscle, bone, or other tissues.
While historically the degree classification has been used, a different classification has also come into common use. Under this alternative classification, superficial burns are the equivalent to first-degree burns. Superficial partial-thickness burns are deeper, extending into the outermost half of the dermal layer. Deep-partialthickness burns pass through the mid-dermal layer and well into the reticular layer. Finally, full-thickness burns are the equivalent to third-degree and fourth-degree burns in that they pass completely through the dermis and into the underlying tissue [10-13].
The advantages of the second classification scheme are that it separately identifies dermal burns into two categories that often have very different treatment avenues. Superficial partial thickness burns will often heal in less than three weeks without significant scarring; however, deep-partial-thickness burns are more likely to require extensive medical care, such as excision and grafting [1-3,5].
Identification of Burns
Accurate identification of scald burns is crucial as this guides subsequent care. Identification of burns involves estimating the TBSA burned location of burns, and depth of burns. It is important to note that burn depth occurs on a continuum and accurate clinical assessment of burn depth is difficult, especially immediately after the injury occurs [3,6-10,13-21].
First degree burns (superficial burns) only damage the epidermis. Burns of this depth are red, have a dry surface, and are often associated with discomfort. They are characterized by a reddening of the skin as the capillaries dilate and bring increased blood flow to the region. The skin will blanch with pressure. Another important distinction is that first degree burns do not blister, but they may lead to sloughing of the epidermal layer. Sunburns are a classic example of first degree burns.
Superficial second degree burns (superficial partial thickness burns) damage the epidermis and superficial dermis. These burns are also painful. Burns of this depth will form blisters, although this may not happen immediately. The fluid within these blisters is clear. Unroofing the blister reveals a moist, pink or red, hypersensitive surface. The surface will blanch with pressure. A pin prick will elicit pain.
Deep second-degree burns (deep partial thickness burns) damage the epidermis and deeper dermal structures including the reticular layer. These burns also will blister. Unroofing the blister will reveal a moist, mottled pink, cherry red or white surface. The pain associated with such burns, may be less than that of superficial partial-thickness burns. There may be decreased sensation to pin prick when compared to undamaged skin. Capillary refill is often reduced or absent.
Differentiating between superficial partial-thickness burns and deep partial thickness burns is especially difficult. Therefore, early after injury such burns have been referred to as intermediate in depth [8].
Third-degree burns (full-thickness burns) extend to the fat or connective tissue layers. Burns of this depth will appear white or tan and will be insensate. These burns do not blanch and will have a dry surface. These burns appear different from the more superficial types because of the complete destruction of the dermis and the blood vessels contained therein. These burns are not painful, because they damage nerves that carry pain signals.
Fourth-degree burns (full-thickness burns) extend through the subcutaneous tissue to deep structures such as muscle and bone. These burns are readily apparent when these deep structures are visible. Figure 1 gives a visual comparison of burn injuries and a summary of characteristics associated with the burns.

Figure 1: Depictions and characteristics of various burn extents (courtesy of Amicus Medical Images).
In addition to burn depth, estimating TBSA burned is important as this guides IV fluid resuscitation. There are several methods for estimating TBSA, but the most commonly used method is the rule of nines [22]. This method is endorsed by the American Burn Association and is used in the Advanced Burn Life Support Manual. For smaller burns, the patient’s hand (palm and fingers) represent approximately 1% TBSA [23]. Figure 2 has been prepared to give a visual and tabulated summary of the rule of nines for both adults and children.
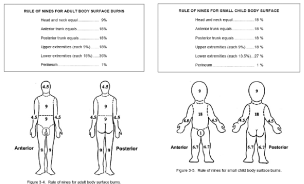
Figure 2: The rule of nines for adults and children (Courtesy of Brookside Associates).
Initial Management and Treatment of Scald Burns
Scald burn injury is very dependent on the liquid temperature and the exposure duration. The impact of these variables on burn depth will be quantified later; however, the strong dependence makes prompt treatment essential. When a scald burn (or any thermal burn) occurs, it is important to remove the source of heat and apply cooling. Delays of even seconds can have a material impact on the ultimate burn depth.
Removal of heat means a separation of the skin from the hot liquid. In some cases, this means a removal of clothing that may be saturated following a spill incident. Saturated clothing has a twopart impact of partially shielding the skin from hot fluid, as well as insulating the skin and helping retain heat after the spill. Depending on the absorbing quality of the clothing, it may hold hot liquid in intimate contact with the skin well after the spill occurrence.
Following the removal of any clothing or other layers that are prolonging the burn insult, the next recommendation is the application of a cool source to the area. Often, cool water is available but other liquids or objects can suffice. The goal is to quickly reduce the skin temperatures. A recommended goal is the application of cooling within 5-10 seconds following the burn. It should also be noted that in an event where cooling is available, such as cool water, it can be applied directly through clothing to speed the cooling process. If the clothing is porous, which most clothing is, the cool liquid will penetrate the cloth nearly instantaneously.
There is extensive literature on the impact of post-burn cooling on human and other mammalian skin. Studies such as [24] have considered the effect of cooling on the microvasculature which in turn influences the post-burn health of the tissue and the tissue regeneration processes. The study, which focused on bat wings, investigated the injury to subcutaneous blood vessels and edema formation. They found that rapid cooling reduced edema, but there were limited long-term benefits. A later study [25] on hamster tissue found that cooling caused an increase in the number of viable blood vessels in the scalded region. This study found that the optimal cooling temperature was in the 5-10oC range, and the cooling was most effective if started immediately. A study using rats found that near immediate irrigation with room temperature water significantly reduced scald injuries [26]; however, the researchers also found that excessively cold temperatures (such as ice) were detrimental. In some instances, ice will bring about full-organism hypothermia, while local application of ice can cause cold-temperature injuries or vasoconstriction and consequently, reduce nutrient flow to the region following the burn. These findings were reinforced in [27] which reported improved clinical and histological assessment following scalding of porcine subjects when cooling was promptly applied. Again in [28] which relied upon hot-beverage spills in children, it was found that removal of saturated clothing and applications of cooling were critical to limit burn injuries.
Subsequent research confirms the above-referenced studies. For instance, partial-thickness scalds in porcine [29] were found to be most effectively treated by the application of cool running water immediately after the burn incident. While [30] also found some positive effect of cooling, it was not as clearly evident as prior work for cooling that is delayed.
Aside from reduction of temperatures following an incident, application of cold has other benefits such as reducing inflammation, edema, and pain [31-35], and these benefits can be realized even after the initial scald temperatures have returned to normothermia.
For serious scalds that require subsequent medical attention (based on the depth of injury, TBSA involved, and/or the area affected) care is best provided at emergency medical centers. A complete review of the management of the burned patient is beyond the scope of this paper, so the focus will be on how burn identification guides initial management and treatment. As mentioned above, the identification of burns informs referral decisions, fluid resuscitation, and wound management.
Burn Center Referral Criteria
The American Burn Association has published burn center referral criteria. Per these criteria, all third degree burns and partial thickness burns involving more than 10% TBSA should be referred to a burn center [1]. Furthermore, burns involving the face, hands, feet, perineum, genitalia, or major joints also warrant referral [1]. A listing of their criteria is provided in Table 1.
Burn Center Referral Criteria
Partial thickness burns greater than 10% of body area
Burns of hands, feet, face, genitals, perineum, or major joints
Third degree burns
Electrical burns (including lightning)
Chemical burns
Inhalation burns
Burns in patients with pre-existing conditions that could complicate management
Concomitant trauma which increases morbidity or mortality risk
Burns of minors treated in hospitals without qualified personnel or equipment
Burn injury which requires social, emotional or rehabilitation intervention
Table 1: Criteria recommended by the American Burn Association for burn center referrals.
Fluid Resuscitation
Burn resuscitation with IV fluids is aimed at ensuring adequate tissue perfusion and preventing hypovolemic shock (burn shock) secondary to fluid loss through damaged skin and fluid accumulation in the interstitial space as edema. First degree burns are not included in the TBSA burn estimation for fluid resuscitation calculations [2-3]. Pediatric patients with less than 10% TBSA burns of second degree or greater can be managed with oral rehydration [3,36]. Likewise, adult patients with less than 20% TBSA burns of second degree or greater can be managed with oral rehydration [36]. However, for partial thickness and full thickness burns involving greater surface area, IV fluid resuscitation with crystalloids is necessary. Ringer’s lactate solution is commonly used [36].
There are several formulas for estimating IV crystalloid needs over the first 24-48 hours, but one of the most popular formulas is the Parkland Formula. To use this formula, one must obtain the patient’s body weight in kilograms (kg) and estimate the TBSA of partial thickness and full thickness burns as described above. This formula estimates how much IV crystalloid is needed in the first 24 hours. Half of the IV fluid is given over the first eight hours, and the remaining half of the IV fluid is given over the last 16 hours. For example, a 70kg patient with 50% TBSA burns of second degree or greater would receive 14 liters of IV fluid over the first 24 hours after the injury with 7 liters being given in the first 8 hours and 7 liters being given over the remaining 16 hours.
Equation 1 – The Parkland Formula:
Milliliters of IV Fluid=4 x TBSA burned as percent x weight in kilograms
Prediction of Burn Depth
As described above, medical care is dictated by the severity of the injury. Insofar as minor burns may require no intervention whereas major burns may require expensive and painful treatments, avoidance of incidents is desired. Here, information will be presented which relates the depth of burns to the temperature of a scalding incident. The focus here will be on information gathered from a series of experiments and numerical simulations on living tissue and on tissue surrogates. The information is directly relevant to other hotliquid spills whose viscosity and heat capacity are of the same order of magnitude as water.
The experiments investigated multiple factors which may influence burn depth. Among them were spill volume, the material which constitutes the cup, the presence or absence of a cap on the cup, and others. It was found that among all the factors investigated, the primary influences are liquid temperature at the time of spill and consequently, that is the focus here. The influence of the other, secondary factors can be attained by reference to [37].
The experiments that were performed had two stages. For the first stage, the goal was to characterize the rate at which beverages cool in a typical ambient environment under various conditions. During the tests, heated water was transferred from a heating vessel to disposable/ compostable beverage cups that were formed using polystyrene or paper. The cups were variously left open to the atmosphere or closed using plastic caps. In addition, corrugated paper sleeves were used on some of the cups to assess the impact of these sleeves on liquid temperature. Multiple experiments were carried out with volumes of liquid that ranged from 8-16 oz (237-473 ml) and statistical analyses were performed. Details of the experiments are provided in [37] and are not repeated here. The outcome of the first set of experiments was a variation of the temperature contained within the beverage container. In addition to the cooling rates, the major finding was that the presence of a disposable cap had a significant impact on the cooling rate of the fluid. Additionally, the volume of hot liquid affected the cooling rate (larger volumes cooled slower). The other parameters, such as composition of the wall, the presence or absence of an insulating sleeve, had negligible impact on the liquid cooling rates and subsequently on scalds.
The second stage of the study involved instrumented living skin and skin-surrogate materials. The heated beverages were purposefully spilled against the surfaces which were covered with a single layer of cotton clothing. Temperatures were measured for thirty second duration and the experiments were repeated to ensure reproducibility. The temperature measurements on living tissue and on the tissue surrogate were mutually reinforcing. It was discovered that the surface temperatures did not depend markedly on the volume of spilled liquid although larger volumes affected a larger area.
The last stage of the study was the input of measured surface temperatures into a numerical model that predicts burn depths. The numerical model was based on the Pennes bio heat transfer equation [38] and was coupled to an injury calculation procedure [39-42]. The methodology presented here has a long history of effectiveness in determining heat transfer within living tissue; [43-61] illustrate both the historical development of the calculation approach as well as the breadth of situations to which the method has been applied.
To provide some specific examples, the model has been successfully applied to calculate the depth of burns from heated liquids with various thermal conditions following the exposure (from insulated to rapid cooling) [62-64]. The research has also lead to the archive of extensive property and tissue thickness information [64], has created simple algebraic equations to estimate burn depths [62,65] and has related burn injury calculations to hyperthermia parameters used in the low-temperature ablation community [66,67]. The results have been thoroughly tested against prior literature and observational evidence of burn depths and have been found to be in excellent agreement.
The results are most simply summarized in Tables 2 and 3 both tables are color-coded according to the burn risk. The table lists both the starting beverage temperatures and the cooling which may occur between service and a spill incident. Red colors are used to indicate temperature/cooling time durations that are likely to lead to deeppartial- thickness burns in adults. Yellow colors indicate temperatures/ cooling times that will likely result in superficial-partial-thickness burns in adults.
Cooling time (minutes)
Service Temperature °C (°F)
70 (158)
75 (167)
80 (176)
85 (185)
90 (194)
95 (203)
0
1
2
3
4
5
6
7
8
9
10
11
12
Table 2: Approximate categorization of burn risk for various service temperatures and cooling durations for cups which are not capped during the cooling time. Red color is used to indicate high risk of deep-partial-thickness burns or worse and yellow indicates high risk of superficial-partial-thickness burns or worse.
Interpretation of Tables 2 and 3 should be done with some care. First, the actual risk of burn depends on the age of the person, their ability to quickly remove the heat source and apply cooling, the thickness of skin, location on the body where the burn occurs, the presence or absence of clothing, the type of clothing, the volume of the spill, and the thermal environment in which the cooling occurs, to name a few.
Readers are reminded that the burn duration for which (Table 3) is generated is 30 seconds. This means that no cooling has been applied within the thirty seconds after a spill. If cooling were applied prior to the 30 second mark, the extent of burn depth would be lessened. On the other hand, these results can be viewed as an upper bound. It is important to note that the actual time a hot beverage is on a victims clothes is accounted for by the experiments which incorporated spill experiments on actually clothing and measurements of the temperature at the skin surface.
Cooling time (minutes)
Service Temperature °C (°F)
70 (158)
75 (167)
80 (176)
85 (185)
90 (194)
95 (203)
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
Table 3: Approximate categorization of burn risk for various service temperatures and cooling durations for cups which are capped during the cooling time. Red color is used to indicate high risk of deep-partial-thickness burns or worse and yellow indicates high risk of superficial-partial-thickness burns or worse.
Despite these potential variations, the below tables are reasonable guidelines to use in practice. They are corroborated with experimental and clinical evidence within a reasonable degree of scientific certainty. These tables can be used as guidelines for situations where heated liquids may spill and cause injury. Special care should be taken for potential spills on children whose skin is thinner than adults and consequently burn at lower temperatures [68-71]. Similarly, special care should be afforded for elderly or persons whose mobility is compromised. These findings also support the general consensus in the scientific literature that often, beverage service temperatures are hotter than consumer preference and pose an unnecessary risk of injury [72-80].
Concluding Remarks
This manuscript brings together information that is useful for first responders, medical practitioners, and engineers to identify, predict, avoid, and provide initial treatment for scald injuries. Accurate identification of burn depth is difficult early after the initial injury. Yet, accurate burn depth identification predicts wound healing. First-degree and superficial partial-thickness burns typically will heal with minimal scaring in less than three weeks. These burns can often be managed with local wound care alone. However, deep partial-thickness burns and full-thickness burns typically form hypertrophic scars and have prolonged healing. Therefore, these more severe burns are often treated by burn surgeons with excision and grafting. Children with more than 10% TBSA partial thickness and full thickness burns and adults with more than 20% TBSA partial thickness and full thickness burns will require IV fluid resuscitation; the Parkland formula can help providers estimate IV crystalloid needs over the first 24 hours after injury. Burn depth, burn location, and TBSA burned help clinicians determine if a burn victim would benefit from referral to a burn center.
The information presented here also can be used as an estimate for the potential of hot-liquid spills to cause scald injuries. The information is taken from multiple sources but is mutually reinforcing. The sources include experiments carried out on animal models and humans, analytical calculations, and advanced numerical simulation. The study includes the effect of cooling time between the service of a beverage and a spill occurrence. Inherent to the information is the effect of cup construction, the presence or absence of a cap, the presence or absence of an insulating sleeve, the volume of the liquid, and the thermal environment.
Conflict of Interest
Dr. Abraham has served as an expert witness in burn litigation.
References
- Advanced Burn Life Support Course Provider Manual, Published by the American Burn Association. 2007.
- Singer AJ, Taira BR, Lee CC. Rosen’s Emergency Medicine. 8th edition. Chapter 63, Thermal Burns. 2014.
- Enoch S, Roshan A, Shah M. Emergency and early management of burns and scalds. BMJ. 2009; 338: b1037.
- Rowan MP, Cancio LC, Elster EA, Burmeister DM, Rose LF, Natesan S. Burn wound healing and treatment: review and advancements. Crit Care. 2015; 19: 243.
- Cubison TC, Pape SA, Parkhouse N. Evidence for the link between healing time and the development of Hypertrophic Scars (HTS) in paediatric burns due to scald injury. Burns. 2006; 32: 992-999.
- Jaskille AD, Ramella-Roman JC, Shupp JW, Jordan MH, Jeng JC. Critical review of burn depth assessment techniques: part II. Review of laser doppler technology. J Burn Care Res. 2010; 31: 151-157.
- Heimbach DM, Afromowitz MA, Engrav LH, Marvin JA, Perry B. Burn depth estimation--man or machine. J Trauma. 1984; 24: 373-378.
- Gibran NS, Giavonni ML, Heimbach DM. Total Burn Care. Evaluation of the Burn Wound: Management Decisions. Chapter 10, Elsevier, 2012.
- Hlava P, Moserova J, Konigova R. Validity of Clinical Assessment of the Depth of a Thermal Injury. Acta Chirurgiae Plasticae. 1983; 25: 202.
- Baxter CR, Waeckerle JF. Emergency treatment of burn injury. Ann Emerg Med. 1988; 17: 1305-1315.
- Ahrenholz DH, Clayton MC, Solem LD. Burns and Wound Management. Wound Healing for the Otolaryn – Head and Neck Surg. 1995: 28; 1039-1054.
- Monafo WW. Initial management of burns. N Engl J Med. 1996; 335: 1581- 1586.
- Durrant CAT, Simpson AR, Williams G. Thermal Injury – The First 24 h. Current Anaest and Crit Care. 2008: 19; 2546-2563.
- Hlava P, Moserová J, Königová R. Validity of clinical assessment of the depth of a thermal injury. Acta Chir Plast. 1983; 25: 202-208.
- Jaskille AD, Shupp JW, Jordan MH, Jeng JC. Critical review of burn depth assessment techniques: Part I. Historical review. J Burn Care Res. 2009; 30: 937-947.
- Kaiser M, Yafi A, Cinat M, Choi B, Durkin AJ. Noninvasive assessment of burn wound severity using optical technology: a review of current and future modalities, Burns. 2011; 37: 377-386.
- Niazi ZB, Essex TJ, Papini R, Scott D, McLean NR, Black MJ. New laser Doppler scanner, a valuable adjunct in burn depth assessment, Burns. 1993; 19: 485-489.
- Jeng JC, Bridgeman A, Shivnan L, Thornton PM, Alam H, Clarke TJ. Laser Doppler imaging determines need for excision and grafting in advance of clinical judgment: a prospective blinded trial. Burns. 2003; 29: 665-670.
- Pape SA, Skouras CA, Byrne PO. An audit of the use of Laser Doppler Imaging (LDI) in the assessment of burns of intermediate depth. Burns. 2001; 27: 233-239.
- Singer AJ, Berutti L, Thode HC, McClain SA. Standardized Burn Model Using Multiparametric Histological Analysis of Burn Depth. Academic Emergency Med. 2000; 7; 1-6.
- Khatib M, Jabir S, Fitzgerald O'Connor E, Philp B. A systematic review of the evolution of laser Doppler techniques in burn depth assessment. Plast Surg Int. 2014; 2014: 621792.
- Jimenez CJ, Mlcak RP, Buffalo MC. Total Burn Care. Pre-hospital Management, Transportation, and Emergency Care. Chapter 7. 2012.
- Sheridan RL, Petras L, Basha G, Salvo P, Cifrino C, Hinson M. Planimetry study of the percent of body surface represented by the hand and palm: sizing irregular burns is more accurately done with the palm. J Burn Care Rehabil. 1995; 16: 605-606.
- Wiedeman MP, Brigham MP. The effects of cooling on the microvasculature after thermal injury. Microvasc Res. 1971; 3: 154-161.
- Ross DC, Diller KR. Therapeutic Effects of Post burn Cooling. J Biomech Eng. 1978; 100: 149-152.
- Sawada Y, Urushidate S, Yotsuyanagi T, Ishita K. Is prolonged and excessive cooling of a scalded wound effective? Burns. 1997; 23: 55-58.
- Jandera V, Hudson DA, de Wet PM, Innes PM, Rode H. Cooling the burn wound: evaluation of different modalites. Burns. 2000; 26: 265-270.
- Nguyen NL, Gun RT, Sparnon AL, Ryan P. The importance of immediate cooling--a case series of childhood burns in Vietnam. Burns. 2002; 28: 173- 176.
- Yuan J, Wu C, Holland AJ, Harvey JG, Martin HC, La Hei ER. Assessment of cooling on an acute scald burn injury in a porcine model. J Burn Care Res. 2007; 28: 514-520.
- Rajan V, Bartlett N, Harvey JG, Martin HC, La Hei ER, Arbuckle S. Delayed cooling of an acute scald contact burn injury in a porcine model: is it worthwhile? J Burn Care Res. 2009; 30: 729-734.
- Davies JW. Prompt cooling of burned areas: a review of benefits and the effectors mechanisms. Burns Incl Therm Inj. 1982; 9: 1-6.
- Jakobsson OP, Arturson G. The effect of prompt local cooling on oedema formation in scalded rat paws. Burns Incl Therm Inj. 1985; 12: 8-15.
- Ernst E, Fialka V. Ice freezes pain? A review of the clinical effectiveness of analgesic cold therapy. J Pain Symptom Manage. 1994; 9: 56-59.
- Wang K, Tavakkoli F, Wang S, Vafai K. Analysis and analytical characterization of bio heat transfer during radiofrequency ablation. J Biomech. 2015; 48: 930- 940.
- Werner MU, Lassen B, Pedersen JL, Kehlet H. Local cooling does not prevent hyperalgesia following burn injury in humans. Pain. 2002; 98: 297-303.
- Pham TN, Cancio LC, Gibran NS; American Burn Association. American Burn Association practice guidelines burn shock resuscitation. J Burn Care Res. 2008; 29: 257-266.
- Abraham JP, Nelson-Cheeseman BB, Sparrow EM, Wentz JE, Gorman JM, Wolf SE. Comprehensive Method to Predict and Quantify Scald Burns from Beverage Spills. Int J Hyperthermia (submitted).
- Pennes HH. Analysis of Tissue and Arterial Blood Temperatures in Resting Human Forearm. J Appl Physiology. 1948; 1: 93-133
- Henriques FC, Moritz AR. Studies of Thermal Injury: I. The Conduction of Heat to and through Skin and the Temperatures Attained Therein. A Theoretical and an Experimental Investigation. Am J Pathol. 1947; 23: 530-549.
- Moritz AR, Henriques FC. Studies of Thermal Injury: II. The Relative Importance of Time and Surface Temperature in the Causation of Cutaneous Burns. Am J Pathol. 1947; 23: 695-720.
- Moritz AR. Studies of Thermal Injury: III. The Pathology and Pathogenesis of Cutaneous Burns. An Experimental Study. Am J Pathol. 1947; 23: 915-941.
- HENRIQUES FC Jr. Studies of thermal injury; the predictability and the significance of thermally induced rate processes leading to irreversible epidermal injury. Arch Pathol (Chic). 1947; 43: 489-502.
- Diller KR. Modeling thermal skin burns on a personal computer. J Burn Care Rehabil. 1998; 19: 420-429.
- Ng EY, Tan HM, Ooi EH. Boundary element method with bioheat equation for skin burn injury. Burns. 2009; 35: 987-997.
- Diller KR, Hayes LJ, Blake GK. Analysis of alternate models for simulating thermal burns. J Burn Care Rehabil. 1991; 12: 177-189.
- Ng EY, Chua LT. Prediction of skin burn injury. Part 1: Numerical modeling. Proc Inst Mech Eng H. 2002; 216: 157-170.
- Ng EY, Chua LT. Prediction of skin burn injury. Part 2: Parametric and sensitivity analysis. Proc Inst Mech Eng H. 2002; 216: 171-183.
- Ng EY, Chua LT. Comparison of one- and two-dimensional programmes for predicting the state of skin burns. Burns. 2002; 28: 27-34.
- Dai W, Wang H, Jordan PM, Mickens RE, Bejan A. A Mathematical Model for Skin Burn Injury Induced by Radiation Heating. Intl J Heat Mass Trans. 2008: 51; 5497-5510.
- Lovik RD, Abraham JP, Sparrow EM. Potential Tissue Damage from Transcutaneous Recharge of Neuromodulation Implants. Intl J Heat Mass Trans. 2009; 52: 3518-3524.
- Smith DK, Lovik RD, Sparrow EM, Abraham JP. Human Tissue Temperatures Achieved During Recharging of New-Generation Neuromodulation Devices. Intl J Heat Mass Trans. 2010; 53: 3292-3299.
- Ross DC, Diller KR. An Experimental Investigation of Burn Injury in Living Tissue. J Heat Trans. 1976; 98: 292-296.
- Diller KR. Modeling of Bioheat Transfer Processes at High and Low Temperature. In Advances in Heat Transfer, 22, Academic Press, New York, 1992.
- Abraham JP, Sparrow EM. A Thermal Ablation Model Including Liquidto- Vapor Phase Change, Necrosis-Dependent Perfusion, and Moisture- Dependent Properties. Intl J Heat Mass Trans. 2007; 50: 2537-2544.
- Weaver JA, Stoll AM. Mathematical model of skin exposed to thermal radiation. Aerosp Med. 1969; 40: 24-30.
- Iasiello M, Vafai K, Andreozzi A, Bianco N, Tavakkoli F. Effects of External and Internal Hyperthermia on LDL Transport and Accumulation Within an Arterial Wall in the Presence of a Stenosis. Ann Biomed Eng. 2015; 43: 1585- 1599.
- Iasiello M, Vafai K, Andreozzi A, Bianco N. Low-Density Lipoprotein Transport through an Artery wall Under Hyper thermal and Hypertension Conditions – An Analytical Solution. J Biomech. 2016; 49: 193-204.
- Gasperin M, Juricić D. The uncertainty in burn prediction as a result of variable skin parameters: an experimental evaluation of burn-protective outfits. Burns. 2009; 35: 970-982.
- Lovik RD, Abraham JP, Sparrow EM. Surrogate Human Tissue Temperatures Resulting from Misalignment of Antenna and Implant during Recharging of a Neuromodulation Device. J Neuromodulation. 2011; 14: 501-511.
- Sparrow EM, Abraham JP, Bayazit Y, Lovik RD, Smith DK. Numerical and Experimental Simulations as Symbiotic Tools for Solving Complex Biothermal Problems. J Med Dev. 2010; 4.
- Lovik RD, Sparrow EM, Abraham JP, Zelmer C, Oh S, Friend K, et al. Effect of Component Misalignment on Human Tissue Temperatures Associated with Recharged Neuromodulation Devices. J Med Dev. 2011; 5.
- Abraham JP, Plourde BD, Vallez LJ, Stark JR, Diller KR. Estimating the Time and Temperature Relationship for Causation of Deep-Partial Thickness Skin Burns. 2015; 41: 1741-1747.
- Abraham JP, Plourde BD, Vallez LJ, Nelson-Cheeseman BB, Stark JR, Gorman JM, et al. Skin Burn, in: Theory and Application of Heat Transfer in Cells and Organs, edited by Devashish Shrivastava, Wiley.
- Johnson NN, Abraham JP, Helgeson ZI, Minkowycz WJ, Sparrow EM. An Archive of Skin-Layer Thicknesses and Properties and Calculations of Scald Burns with Comparisons to Experimental Observations. J Thermal Sci Engr Appln. 2011; 3.
- Abraham JP, Hennessey MP, Minkowycz WJ. A Simple Algebraic Model to Predict Burn Depth and Injury. Intl Comm Heat Mass Trans. 2011; 38: 1169- 1171.
- Viglianti BL, Dewhirst MW, Abraham JP, Gorman JM, Sparrow EM. Rationalization of thermal injury quantification methods: application to skin burns. Burns. 2014; 40: 896-902.
- Dewhirst MW, Abraham JP, Viglianti BL. Evolution of Thermal Dosimetry for Applications of Hyperthermia Treatment to Cancer, in: Advances in Heat Transfer. 2015: 47; 397-421.
- Huzaira M, Rius F, Rajadhyaksha M, Anderson RR, González S. Topographic variations in normal skin, as viewed by in vivo reflectance confocal microscopy. J Invest Dermatol. 2001; 116: 846-852.
- Seidenari S, Giusti G, Bertoni L, Magnoni C, Pellacani G. Thickness and echogenicity of the skin in children as assessed by 20-MHz ultrasound. Dermatology. 2000; 201: 218-222.
- Conti A, Schiavai ME, Seidenari S. Capacitance, Transepidermal Water Loss and Causal Level of Sebum in Healthy Subjects in Relation to Site, Sex and Age. Intl J Cosmetic Sci. 1995; 17: 77-85.
- Diller KR. Adapting adult scald safety standards to children. J Burn Care Res. 2006; 27: 314-322.
- Ramanathan C, Ekpenyong L, Stevenson JH. Scald burns in children caused by hot drinks--the importance of the type of cup. Burns. 1994; 20: 111-114.
- Mercer NS. With or without? A cooling study. Burns Incl Therm Inj. 1988; 14: 397-398.
- Warner RM, Wilson Y, Chester DL. Cooling properties of everyday liquids. Burns. 2012; 38: 1186-1191.
- Brown F, Diller KR. calculating the optimum temperature for serving hot beverages. Burns. 2008; 34: 648-654.
- Pipatsattayanutong S, Lee HS, Lau S, O’Mahony M. Hedonic R-Index Measurement of Temperature Preferences for Drinking Black Coffee. J Sensor Studies. 2001; 16: 517-536.
- Borchgrevink CP, Susskind AM, Tarras JT. Consumer Preferred Hot Beverage Temperatures. Food Quality and Preference. 1999; 10: 117-121.
- Lee HS, O’Mahony M. At What Temperature do Consumers Like to Drink Coffee?: Mixing Methods, J Food Science. 2002; 67: 2774-2777.
- Jamnadas-Khoda B, See MS, Cubison CT, Dheansa BS. How would you like your tea, vicar? Burns. 2010; 36: 356-359.
- Abraham JP, Plourde BD, Vallez LJ, Nelson-Cheeseman BB. Correcting a prevalent misunderstanding of burns. Burns. 2016; 42: 715-716.